Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis
- PMID: 23610413
- PMCID: PMC3651482
- DOI: 10.1073/pnas.1300725110
Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis
Abstract
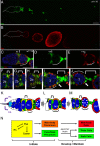
Asymmetric division of stem cells results in both self-renewal and differentiation of daughters. Understanding the molecules and mechanisms that govern differentiation of specific cell types from adult tissue stem cells is a major challenge in developmental biology and regenerative medicine. Drosophila follicle stem cells (FSCs) represent an excellent model system to study adult stem cell behavior; however, the earliest stages of follicle cell differentiation remain largely mysterious. Here we identify Castor (Cas) as a nuclear protein that is expressed in FSCs and early follicle cell precursors and then is restricted to differentiated polar and stalk cells once egg chambers form. Cas is required for FSC maintenance and polar and stalk cell fate specification. Eyes absent (Eya) is excluded from polar and stalk cells and represses their fate by inhibiting Cas expression. Hedgehog signaling is essential to repress Eya to allow Cas expression in polar and stalk cells. Finally, we show that the complementary patterns of Cas and Eya reveal the gradual differentiation of polar and stalk precursor cells at the earliest stages of their development. Our studies provide a marker for cell fates in this model and insight into the molecular and cellular mechanisms by which FSC progeny diverge into distinct fates.
Keywords: germarium; polar cell; polar stalk precursor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Spradling AC. Developmental genetics of oogenesis. In: Martinez-Arias B, editor. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. pp. 1–70.
-
- Grammont M, Irvine KD. Fringe and Notch specify polar cell fate during Drosophila oogenesis. Development. 2001;128(12):2243–2253. - PubMed
-
- Grammont M, Irvine KD. Organizer activity of the polar cells during Drosophila oogenesis. Development. 2002;129(22):5131–5140. - PubMed
-
- Vachias C, Couderc JL, Grammont M. A two-step Notch-dependant mechanism controls the selection of the polar cell pair in Drosophila oogenesis. Development. 2010;137(16):2703–2711. - PubMed
-
- Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev Cell. 2003;4(2):167–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

