Geometric control of vascular networks to enhance engineered tissue integration and function
- PMID: 23610423
- PMCID: PMC3651499
- DOI: 10.1073/pnas.1217796110
Geometric control of vascular networks to enhance engineered tissue integration and function
Abstract
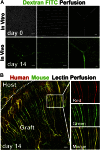
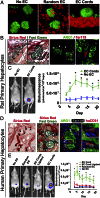
Tissue vascularization and integration with host circulation remains a key barrier to the translation of engineered tissues into clinically relevant therapies. Here, we used a microtissue molding approach to demonstrate that constructs containing highly aligned "cords" of endothelial cells triggered the formation of new capillaries along the length of the patterned cords. These vessels became perfused with host blood as early as 3 d post implantation and became progressively more mature through 28 d. Immunohistochemical analysis showed that the neovessels were composed of human and mouse endothelial cells and exhibited a mature phenotype, as indicated by the presence of alpha-smooth muscle actin-positive pericytes. Implantation of cords with a prescribed geometry demonstrated that they provided a template that defined the neovascular architecture in vivo. To explore the utility of this geometric control, we implanted primary rat and human hepatocyte constructs containing randomly organized endothelial networks vs. ordered cords. We found substantially enhanced hepatic survival and function in the constructs containing ordered cords following transplantation in mice. These findings demonstrate the importance of multicellular architecture in tissue integration and function, and our approach provides a unique strategy to engineer vascular architecture.
Keywords: angiogenesis; liver; regenerative medicine; tissue engineering; vascular biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vacanti JP, Langer R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–SI34. - PubMed
-
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. - PubMed
-
- Folkman J. Looking for a good endothelial address. Cancer Cell. 2002;1(2):113–115. - PubMed
-
- Radisic M, et al. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82(4):403–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

