Soluble IL7Rα potentiates IL-7 bioactivity and promotes autoimmunity
- PMID: 23610432
- PMCID: PMC3651437
- DOI: 10.1073/pnas.1222303110
Soluble IL7Rα potentiates IL-7 bioactivity and promotes autoimmunity
Abstract
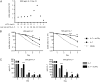
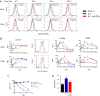
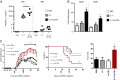
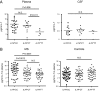
Human soluble interleukin-7 receptor (sIL7R)α circulates in high molar excess compared with IL-7, but its biology remains unclear. We demonstrate that sIL7Rα has moderate affinity for IL-7 but does not bind thymic stromal lymphopoietin. Functionally, sIL7Rα competes with cell-associated IL-7 receptor to diminish excessive IL-7 consumption and, thus, enhances the bioactivity of IL-7 when the cytokine is limited, as it is presumed to be in vivo. IL-7 signaling in the presence of sIL7Rα also diminishes expression of CD95 and suppressor of cytokine signaling 1, both regulatory molecules. Murine models confirm diminished consumption of IL-7 in the presence of sIL7Rα and also demonstrate a potentiating effect of sIL7Rα on IL-7-mediated homeostatic expansion and experimental autoimmune encephalomyelitis exacerbation. In multiple sclerosis and several other autoimmune diseases, IL7R genotype influences susceptibility. We measured increased sIL7Rα levels, as well as increased IL-7 levels, in multiple sclerosis patients with the predisposing IL7R genotype, consistent with diminished IL-7 consumption in vivo. This work demonstrates that sIL7Rα potentiates IL-7 bioactivity and provides a basis to explain the increased risk of autoimmunity observed in individuals with genotype-induced elevations of sIL7Rα.
Keywords: immunology; soluble receptors; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. - PubMed
-
- Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. - PubMed
-
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11(2):173–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

