Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology
- PMID: 23610565
- PMCID: PMC3617971
- DOI: 10.1159/000346180
Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology
Abstract
Background: Genetically modified mice are used to investigate disease and assess potential interventions. However, research into kidney fibrosis is hampered by a lack of models of chronic kidney disease (CKD) in mice. Recently, aristolochic acid nephropathy (AAN), characterised by severe tubulointerstitial fibrosis, has been identified as a cause of end stage kidney disease and proposed as a model of CKD. Published studies have used various dosing regimens, species and strains, with variable outcomes. Therefore, we aimed to develop a standardised protocol to develop tubulointerstitial fibrosis using pure aristolochic acid I (AAI) in C57BL/6 mice.
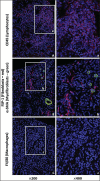
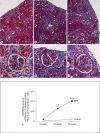
Methods: AAI dose optimisation was performed by intraperitoneal injection of AAI at varying dose, frequency and duration. Kidney function was assessed by serum creatinine. Fibrosis was quantified by hydroxyproline levels and Masson's Trichrome staining. Specific collagens were measured by immunofluorescent staining.
Results: Single doses of AAI of >10 mg/kg caused acute kidney failure and death. Lower doses of 2.5 mg/kg needed to be administrated more than weekly to cause significant fibrosis. 3 mg/kg once every 3 days for 6 weeks followed by a disease development time of 6 weeks after AAI led to reduced kidney weight and function. Substantial tubulointerstitial fibrosis occurred, with males more severely affected. Increased deposition of collagen I, III and IV contributed to fibrosis, with collagen III and IV higher in males.
Conclusions: AAN can be induced in C57BL/6 mice. The regimen of 3 mg/kg every 3 days for 6 weeks followed by 6 weeks of disease development time gives substantial tubulointerstitial fibrosis with lesions similar to those in humans.
Keywords: Aristolochic acid nephropathy; C57BL/6 mice; Chronic kidney disease; Extracellular matrix; Fibrosis.
Figures








References
-
- Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90:87–92. - PubMed
-
- Pacholczyk G, Suhag R, Mazurek M, Dederscheck SM, Koni PA. Generation of C57BL/6 knockout mice using C3H × BALB/c blastocysts. Biotechniques. 2008;44:413–416. - PubMed
-
- Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 2004;20:59–62. - PubMed
-
- Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlondorff D. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

