The horse as a model of naturally occurring osteoarthritis
- PMID: 23610661
- PMCID: PMC3626203
- DOI: 10.1302/2046-3758.111.2000132
The horse as a model of naturally occurring osteoarthritis
Abstract
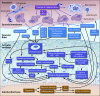
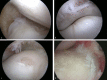
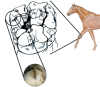
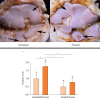
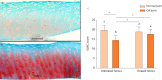
Osteoarthritis (OA) is an important cause of pain, disability and economic loss in humans, and is similarly important in the horse. Recent knowledge on post-traumatic OA has suggested opportunities for early intervention, but it is difficult to identify the appropriate time of these interventions. The horse provides two useful mechanisms to answer these questions: 1) extensive experience with clinical OA in horses; and 2) use of a consistently predictable model of OA that can help study early pathobiological events, define targets for therapeutic intervention and then test these putative therapies. This paper summarises the syndromes of clinical OA in horses including pathogenesis, diagnosis and treatment, and details controlled studies of various treatment options using an equine model of clinical OA.
Keywords: Diagnosis; Equine; Experimental model; Model; Osteoarthritis; Therapy.
Conflict of interest statement
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

