Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells
- PMID: 23611188
- PMCID: PMC4209492
- DOI: 10.1089/cbr.2012.1329
Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells
Abstract
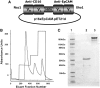
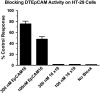
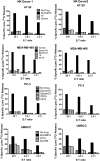
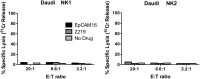
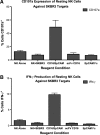
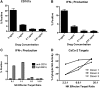
A heterodimeric bispecific biological recombinant drug was synthesized by splicing DNA fragments from two fully humanized single-chain variable-fragment (scFV) antibody fragments forming a novel drug simultaneously recognizing the CD16 natural killer (NK) cell marker and the cancer marker epithelial cell adhesion molecule (EpCAM). The drug precipitously enhanced the killing of human carcinomas of the prostate, breast, colon, head, and neck even at very low effector:target ratios. The drug EpCAM16 rendered even nonactivated NK cell-proficient killers and activated them to kill via degranulation and cytokine production. Studies show that bispecific antibodies can be used to induce proficient killing of the carcinoma targets that ordinarily are resistant to NK-mediated killing. Apparently, the innate immune system can be effectively recruited to kill cancer cells using the bispecific antibody platform and EpCAM targeting.
Keywords: ADCC; anti-CD16; bispecific antibody; carcinoma; human NK cells.
Figures

Similar articles
-
Heterodimeric Bispecific Single Chain Variable Fragments (scFv) Killer Engagers (BiKEs) Enhance NK-cell Activity Against CD133+ Colorectal Cancer Cells.Target Oncol. 2016 Jun;11(3):353-61. doi: 10.1007/s11523-015-0391-8. Target Oncol. 2016. PMID: 26566946 Free PMC article.
-
Tetraspecific scFv construct provides NK cell mediated ADCC and self-sustaining stimuli via insertion of IL-15 as a cross-linker.Oncotarget. 2016 Nov 8;7(45):73830-73844. doi: 10.18632/oncotarget.12073. Oncotarget. 2016. PMID: 27650544 Free PMC article.
-
Enhanced ADCC and NK Cell Activation of an Anticarcinoma Bispecific Antibody by Genetic Insertion of a Modified IL-15 Cross-linker.Mol Ther. 2016 Aug;24(7):1312-22. doi: 10.1038/mt.2016.88. Epub 2016 May 9. Mol Ther. 2016. PMID: 27157665 Free PMC article.
-
[Bispecific antibodies: An old story with a bright future… with CAR-T cells!].Bull Cancer. 2021 Oct;108(10S):S168-S180. doi: 10.1016/j.bulcan.2021.02.016. Bull Cancer. 2021. PMID: 34920800 Review. French.
-
Catumaxomab: clinical development and future directions.MAbs. 2010 Mar-Apr;2(2):129-36. doi: 10.4161/mabs.2.2.11221. MAbs. 2010. PMID: 20190561 Free PMC article. Review.
Cited by
-
Killers 2.0: NK cell therapies at the forefront of cancer control.J Clin Invest. 2019 Sep 3;129(9):3499-3510. doi: 10.1172/JCI129338. J Clin Invest. 2019. PMID: 31478911 Free PMC article. Review.
-
A vector-encoded bispecific killer engager to harness virus-activated NK cells as anti-tumor effectors.Cell Death Dis. 2023 Feb 10;14(2):104. doi: 10.1038/s41419-023-05624-3. Cell Death Dis. 2023. PMID: 36765035 Free PMC article.
-
Natural killer cells in antitumour adoptive cell immunotherapy.Nat Rev Cancer. 2022 Oct;22(10):557-575. doi: 10.1038/s41568-022-00491-0. Epub 2022 Jul 25. Nat Rev Cancer. 2022. PMID: 35879429 Free PMC article. Review.
-
NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy.Front Immunol. 2015 Jul 27;6:368. doi: 10.3389/fimmu.2015.00368. eCollection 2015. Front Immunol. 2015. PMID: 26284063 Free PMC article. Review.
-
Proceedings from the Second Haploidentical Stem Cell Transplantation Symposium-Haplo2014, San Francisco, California, December 4, 2014.Biol Blood Marrow Transplant. 2016 Apr;22(4):594-604. doi: 10.1016/j.bbmt.2016.01.001. Epub 2016 Jan 13. Biol Blood Marrow Transplant. 2016. PMID: 26806585 Free PMC article.
References
-
- Litvinov SV. Bakker HA. Gourevitch MM, et al. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994;2:417. - PubMed
-
- Munz M. Baeuerle PA. Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

