The osteocyte: an endocrine cell ... and more
- PMID: 23612223
- PMCID: PMC3785641
- DOI: 10.1210/er.2012-1026
The osteocyte: an endocrine cell ... and more
Abstract
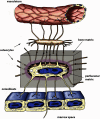
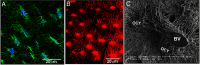
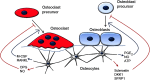
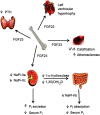
Few investigators think of bone as an endocrine gland, even after the discovery that osteocytes produce circulating fibroblast growth factor 23 that targets the kidney and potentially other organs. In fact, until the last few years, osteocytes were perceived by many as passive, metabolically inactive cells. However, exciting recent discoveries have shown that osteocytes encased within mineralized bone matrix are actually multifunctional cells with many key regulatory roles in bone and mineral homeostasis. In addition to serving as endocrine cells and regulators of phosphate homeostasis, these cells control bone remodeling through regulation of both osteoclasts and osteoblasts, are mechanosensory cells that coordinate adaptive responses of the skeleton to mechanical loading, and also serve as a manager of the bone's reservoir of calcium. Osteocytes must survive for decades within the bone matrix, making them one of the longest lived cells in the body. Viability and survival are therefore extremely important to ensure optimal function of the osteocyte network. As we continue to search for new therapeutics, in addition to the osteoclast and the osteoblast, the osteocyte should be considered in new strategies to prevent and treat bone disease.
Figures

References
-
- Beno T, Yoon YJ, Cowin SC, Fritton SP. Estimation of bone permeability using accurate microstructural measurements. J Biomech. 2006;39(13):2378–2387 - PubMed
-
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–190 - PubMed
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–137 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

