Metal ion and inter-domain interactions as functional networks in E. coli topoisomerase I
- PMID: 23612251
- PMCID: PMC3876943
- DOI: 10.1016/j.gene.2013.04.008
Metal ion and inter-domain interactions as functional networks in E. coli topoisomerase I
Abstract
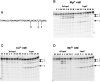
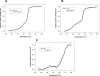
Escherichia coli topoisomerase I (EcTopoI) is a type IA bacterial topoisomerase which is receiving large attention due to its potential application as novel target for antibacterial therapeutics. Nevertheless, a detailed knowledge of its mechanism of action at molecular level is to some extent lacking. This is partly due to the requirement of several factors (metal ions, nucleic acid) to the proper progress of the enzyme catalytic cycle. Additionally, each of them can differently affect the protein structure. Here we assess the role of the different components (DNA, metal ions, protein domains) in a dynamic environment as in solution by monitoring the catalytic as well as the structural properties of EcTopoI. Our results clearly indicated the interaction among these components as functionally relevant and underlined their mutual involvement. Some similarities with other enzymes of the same family emerged (for example DNA prevents divalent metal ions coordination at non selective binding sites). Interestingly, same interactions (C- and N-terminal domain interaction) appear to be peculiar of this bacterial topoisomerase which suggest they could be favorably exploited to the design of selective inhibitors for this class of enzyme.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

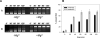





Similar articles
-
The strictly conserved Arg-321 residue in the active site of Escherichia coli topoisomerase I plays a critical role in DNA rejoining.J Biol Chem. 2011 May 27;286(21):18673-80. doi: 10.1074/jbc.M111.229450. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21478161 Free PMC article.
-
Crystal structure of a covalent intermediate in DNA cleavage and rejoining by Escherichia coli DNA topoisomerase I.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6939-44. doi: 10.1073/pnas.1100300108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482796 Free PMC article.
-
Deciphering the distinct role for the metal coordination motif in the catalytic activity of Mycobacterium smegmatis topoisomerase I.J Mol Biol. 2009 Nov 6;393(4):788-802. doi: 10.1016/j.jmb.2009.08.064. Epub 2009 Sep 3. J Mol Biol. 2009. PMID: 19733176
-
Effects of magnesium and related divalent metal ions in topoisomerase structure and function.Nucleic Acids Res. 2009 Feb;37(3):702-11. doi: 10.1093/nar/gkp024. Epub 2009 Feb 2. Nucleic Acids Res. 2009. PMID: 19188255 Free PMC article. Review.
-
Bacterial and archeal type I topoisomerases.Biochim Biophys Acta. 1998 Oct 1;1400(1-3):19-27. doi: 10.1016/s0167-4781(98)00125-0. Biochim Biophys Acta. 1998. PMID: 9748482 Review.
Cited by
-
Iron and zinc binding activity of Escherichia coli topoisomerase I homolog YrdD.Biometals. 2014 Apr;27(2):229-36. doi: 10.1007/s10534-013-9698-z. Epub 2014 Jan 29. Biometals. 2014. PMID: 24469504 Free PMC article.
-
Microheterogeneity of Topoisomerase IA/IB and Their DNA-Bound States.ACS Omega. 2019 Feb 28;4(2):3619-3626. doi: 10.1021/acsomega.8b02887. Epub 2019 Feb 18. ACS Omega. 2019. PMID: 30842985 Free PMC article.
-
Synthetic studies on the reverse antibiotic natural products, the nybomycins.Medchemcomm. 2019 May 24;10(8):1438-1444. doi: 10.1039/c9md00207c. eCollection 2019 Aug 1. Medchemcomm. 2019. PMID: 31534658 Free PMC article.
-
A Gold Nanoparticle Nanonuclease Relying on a Zn(II) Mononuclear Complex.Angew Chem Int Ed Engl. 2021 Jan 18;60(3):1423-1432. doi: 10.1002/anie.202012513. Epub 2020 Nov 16. Angew Chem Int Ed Engl. 2021. PMID: 32985766 Free PMC article.
-
Duplex DNA and BLM regulate gate opening by the human TopoIIIα-RMI1-RMI2 complex.Nat Commun. 2022 Jan 31;13(1):584. doi: 10.1038/s41467-022-28082-5. Nat Commun. 2022. PMID: 35102151 Free PMC article.
References
-
- Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41:41–101. - PubMed
-
- Podobnik M, McInerney P, O'Donnell M, Kuriyan J. A TOPRIM domain in the crystal structure of the catalytic core of Escherichia coli primase confirms a structural link to DNA topoisomerases. J Mol Biol. 2000;300:353–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

