Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice
- PMID: 23612313
- PMCID: PMC3644083
- DOI: 10.1038/ncomms2718
Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice
Abstract
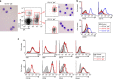
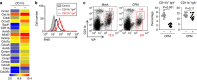
Intestinal plasma cells predominantly produce immunoglobulin (Ig) A, however, their functional diversity remains poorly characterized. Here we show that murine intestinal IgA plasma cells can be newly classified into two populations on the basis of CD11b expression, which cannot be discriminated by currently known criteria such as general plasma cell markers, B cell origin and T cell dependence. CD11b(+) IgA(+) plasma cells require the lymphoid structure of Peyer's patches, produce more IgA than CD11b(-) IgA(+) plasma cells, proliferate vigorously, and require microbial stimulation and IL-10 for their development and maintenance. These features allow CD11b(+) IgA(+) plasma cells to mediate early-phase antigen-specific intestinal IgA responses induced by oral immunization with protein antigen. These findings reveal the functional diversity of IgA(+) plasma cells in the murine intestine.
Figures




Similar articles
-
Migratory CD103+CD11b+ cDC2s in Peyer's patches are critical for gut IgA responses following oral immunization.Mucosal Immunol. 2024 Aug;17(4):509-523. doi: 10.1016/j.mucimm.2024.03.004. Epub 2024 Mar 15. Mucosal Immunol. 2024. PMID: 38492746
-
Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer's patches for intestinal IgA responses.J Immunol. 2008 Apr 15;180(8):5335-43. doi: 10.4049/jimmunol.180.8.5335. J Immunol. 2008. PMID: 18390715
-
CD47-deficient mice have decreased production of intestinal IgA following oral immunization but a maintained capacity to induce oral tolerance.Immunology. 2012 Mar;135(3):236-44. doi: 10.1111/j.1365-2567.2011.03536.x. Immunology. 2012. PMID: 22070457 Free PMC article.
-
[The intestines as an immune organ].Z Gastroenterol Verh. 1987 Apr;22:50-5. Z Gastroenterol Verh. 1987. PMID: 2442909 Review. German. No abstract available.
-
Role of interleukin-6 in human and mouse mucosal IgA plasma cell responses.Immunol Res. 1991;10(3-4):418-22. doi: 10.1007/BF02919734. Immunol Res. 1991. PMID: 1955769 Review.
Cited by
-
Integrin CD11b Negatively Regulates B Cell Receptor Signaling to Shape Humoral Response during Immunization and Autoimmunity.J Immunol. 2021 Oct 1;207(7):1785-1797. doi: 10.4049/jimmunol.2100070. Epub 2021 Sep 1. J Immunol. 2021. PMID: 34470858 Free PMC article.
-
Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity.Mucosal Immunol. 2018 May;11(3):693-702. doi: 10.1038/mi.2017.103. Epub 2018 Feb 7. Mucosal Immunol. 2018. PMID: 29411777
-
Regulation of the immune system by the resident intestinal bacteria.Gastroenterology. 2014 May;146(6):1477-88. doi: 10.1053/j.gastro.2014.01.060. Epub 2014 Feb 4. Gastroenterology. 2014. PMID: 24503128 Free PMC article. Review.
-
Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation.Cell Host Microbe. 2020 Mar 11;27(3):467-475.e6. doi: 10.1016/j.chom.2020.01.016. Epub 2020 Feb 18. Cell Host Microbe. 2020. PMID: 32075742 Free PMC article.
-
Integrin CD11b provides a new marker of pre-germinal center IgA+ B cells in murine Peyer's patches.Int Immunol. 2022 Apr 20;34(5):249-262. doi: 10.1093/intimm/dxab113. Int Immunol. 2022. PMID: 34971392 Free PMC article.
References
-
- Macpherson A. J., McCoy K. D., Johansen F. E. & Brandtzaeg P.. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008). - PubMed
-
- Brandtzaeg P.. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355 (2010). - PubMed
-
- Fagarasan S.. Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Curr. Top Microbiol. Immunol. 308, 137–153 (2006). - PubMed
-
- Macpherson A. J. & Slack E.. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr. Opin. Gastroenterol. 23, 673–678 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

