TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification
- PMID: 23612498
- PMCID: PMC3824607
- DOI: 10.1016/j.cellsig.2013.04.005
TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification
Abstract
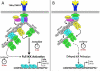
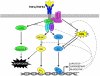
TNFα is a pleotropic cytokine that plays a central role in the inflammatory response by activating the NF-κB signaling pathway, and is targeted in a range of chronic inflammatory diseases, underscoring the therapeutic importance of understanding its underlying molecular mechanisms. Although K63-linked ubiquitination of RIP1 by TRAF2/5 and cIAP1/2 was thought to serve as a scaffold to activate the NF-κB pathway, the recent accumulation of conflicting results has challenged the necessity of these proteins in NF-κB activation. In addition, several serine/threonine kinases have been implicated in TNFα-induced IKK activation; however, the targeted disruption of these kinases had no effect on transient IKK activation. The recent discovery of RIP1-dependent and -independent activation of the early and delayed phases of IKK and TRAF2 phosphorylation-dependent activation of the prolonged phase of IKK offers a reconciliatory model for the interpretation of contradictory results in the field. Notably, the TNFα-induced inflammatory response is not exclusively controlled by the NF-κB pathway but is subject to regulatory crosstalk between NF-κB and other context-dependent pathways. Thus further elucidation of these spatiotemporally-coordinated signaling mechanisms has the potential to provide novel molecular targets and therapeutic strategies for NF-κB intervention.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
TRAIL activates JNK and NF-κB through RIP1-dependent and -independent pathways.Cell Signal. 2015 Feb;27(2):306-14. doi: 10.1016/j.cellsig.2014.11.014. Epub 2014 Nov 18. Cell Signal. 2015. PMID: 25446254 Free PMC article.
-
Two coordinated mechanisms underlie tumor necrosis factor alpha-induced immediate and delayed IκB kinase activation.Mol Cell Biol. 2013 May;33(10):1901-15. doi: 10.1128/MCB.01416-12. Epub 2013 Mar 4. Mol Cell Biol. 2013. PMID: 23459942 Free PMC article.
-
TRAF2 suppresses basal IKK activity in resting cells and TNFalpha can activate IKK in TRAF2 and TRAF5 double knockout cells.J Mol Biol. 2009 Jun 12;389(3):495-510. doi: 10.1016/j.jmb.2009.04.054. Epub 2009 May 3. J Mol Biol. 2009. PMID: 19409903 Free PMC article.
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Taurine protects against perfluorooctanoic acid-induced hepatotoxicity via inhibition of oxidative stress, inflammatory, and apoptotic pathways.Toxicol Res (Camb). 2023 Jan 31;12(1):124-132. doi: 10.1093/toxres/tfad005. eCollection 2023 Feb. Toxicol Res (Camb). 2023. PMID: 36866213 Free PMC article.
-
NF-κB Dynamics Discriminate between TNF Doses in Single Cells.Cell Syst. 2017 Dec 27;5(6):638-645.e5. doi: 10.1016/j.cels.2017.10.011. Epub 2017 Nov 8. Cell Syst. 2017. PMID: 29128333 Free PMC article.
-
Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer.Cancers (Basel). 2020 Dec 15;12(12):3784. doi: 10.3390/cancers12123784. Cancers (Basel). 2020. PMID: 33334024 Free PMC article.
-
Cholesterol restricts lymphotoxin β receptor-triggered NF-κB signaling.Cell Commun Signal. 2019 Dec 26;17(1):171. doi: 10.1186/s12964-019-0460-1. Cell Commun Signal. 2019. PMID: 31878945 Free PMC article.
-
Key oncogenic signaling pathways affecting tumor-infiltrating lymphocytes infiltration in hepatocellular carcinoma: basic principles and recent advances.Front Immunol. 2024 Feb 15;15:1354313. doi: 10.3389/fimmu.2024.1354313. eCollection 2024. Front Immunol. 2024. PMID: 38426090 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

