HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex
- PMID: 23612978
- PMCID: PMC3668709
- DOI: 10.1074/jbc.M112.416735
HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex
Abstract
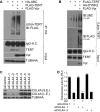
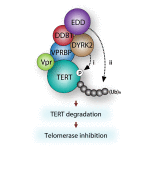
Viral pathogens utilize host cell machinery for their benefits. Herein, we identify that HIV-1 Vpr (viral protein R) negatively modulates telomerase activity. Telomerase enables stem and cancer cells to evade cell senescence by adding telomeric sequences to the ends of chromosomes. We found that Vpr inhibited telomerase activity by down-regulating TERT protein, a catalytic subunit of telomerase. As a molecular adaptor, Vpr enhanced the interaction between TERT and the VPRBP substrate receptor of the DYRK2-associated EDD-DDB1-VPRBP E3 ligase complex, resulting in increased ubiquitination of TERT. In contrast, the Vpr mutant identified in HIV-1-infected long-term nonprogressors failed to promote TERT destabilization. Our results suggest that Vpr inhibits telomerase activity by hijacking the host E3 ligase complex, and we propose the novel molecular mechanism of telomerase deregulation in possibly HIV-1 pathogenesis.
Keywords: DYRK2; EDD; HIV-1; Protein Degradation; TERT; Telomerase; Telomeres; Ubiquitination; VPRBP; Vpr.
Figures




Similar articles
-
Dyrk2-associated EDD-DDB1-VprBP E3 ligase inhibits telomerase by TERT degradation.J Biol Chem. 2013 Mar 8;288(10):7252-62. doi: 10.1074/jbc.M112.416792. Epub 2013 Jan 28. J Biol Chem. 2013. PMID: 23362280 Free PMC article.
-
HIV-1 Vpr hijacks EDD-DYRK2-DDB1DCAF1 to disrupt centrosome homeostasis.J Biol Chem. 2018 Jun 15;293(24):9448-9460. doi: 10.1074/jbc.RA117.001444. Epub 2018 May 3. J Biol Chem. 2018. PMID: 29724823 Free PMC article.
-
HIV-1 Vpr Protein Enhances Proteasomal Degradation of MCM10 DNA Replication Factor through the Cul4-DDB1[VprBP] E3 Ubiquitin Ligase to Induce G2/M Cell Cycle Arrest.J Biol Chem. 2015 Jul 10;290(28):17380-9. doi: 10.1074/jbc.M115.641522. Epub 2015 Jun 1. J Biol Chem. 2015. PMID: 26032416 Free PMC article.
-
CRL4-DCAF1 Ubiquitin Ligase Dependent Functions of HIV Viral Protein R and Viral Protein X.Viruses. 2024 Aug 17;16(8):1313. doi: 10.3390/v16081313. Viruses. 2024. PMID: 39205287 Free PMC article. Review.
-
Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review.
Cited by
-
VprBP (DCAF1): a promiscuous substrate recognition subunit that incorporates into both RING-family CRL4 and HECT-family EDD/UBR5 E3 ubiquitin ligases.BMC Mol Biol. 2013 Sep 13;14:22. doi: 10.1186/1471-2199-14-22. BMC Mol Biol. 2013. PMID: 24028781 Free PMC article. Review.
-
An OTU deubiquitinating enzyme in Eimeria tenella interacts with Eimeria tenella virus RDRP.Parasit Vectors. 2018 Jan 31;11(1):74. doi: 10.1186/s13071-018-2626-x. Parasit Vectors. 2018. PMID: 29386062 Free PMC article.
-
HIV-1 Vpr Functions in Primary CD4+ T Cells.Viruses. 2024 Mar 9;16(3):420. doi: 10.3390/v16030420. Viruses. 2024. PMID: 38543785 Free PMC article. Review.
-
Degradation of SAMHD1 by Vpx Is Independent of Uncoating.J Virol. 2015 May;89(10):5701-13. doi: 10.1128/JVI.03575-14. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762741 Free PMC article.
-
UBR5 promotes antiviral immunity by disengaging the transcriptional brake on RIG-I like receptors.Nat Commun. 2024 Jan 26;15(1):780. doi: 10.1038/s41467-024-45141-1. Nat Commun. 2024. PMID: 38278841 Free PMC article.
References
-
- Margottin F., Bour S. P., Durand H., Selig L., Benichou S., Richard V., Thomas D., Strebel K., Benarous R. (1998) A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 - PubMed
-
- Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X. F. (2003) Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302, 1056–1060 - PubMed
-
- Marin M., Rose K. M., Kozak S. L., Kabat D. (2003) HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9, 1398–1403 - PubMed
-
- Sheehy A. M., Gaddis N. C., Malim M. H. (2003) The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9, 1404–1407 - PubMed
-
- Mariani R., Chen D., Schröfelbauer B., Navarro F., König R., Bollman B., Münk C., Nymark-McMahon H., Landau N. R. (2003) Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114, 21–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

