HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex
- PMID: 23612978
- PMCID: PMC3668709
- DOI: 10.1074/jbc.M112.416735
HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex
Abstract
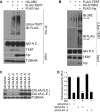
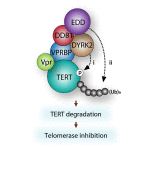
Viral pathogens utilize host cell machinery for their benefits. Herein, we identify that HIV-1 Vpr (viral protein R) negatively modulates telomerase activity. Telomerase enables stem and cancer cells to evade cell senescence by adding telomeric sequences to the ends of chromosomes. We found that Vpr inhibited telomerase activity by down-regulating TERT protein, a catalytic subunit of telomerase. As a molecular adaptor, Vpr enhanced the interaction between TERT and the VPRBP substrate receptor of the DYRK2-associated EDD-DDB1-VPRBP E3 ligase complex, resulting in increased ubiquitination of TERT. In contrast, the Vpr mutant identified in HIV-1-infected long-term nonprogressors failed to promote TERT destabilization. Our results suggest that Vpr inhibits telomerase activity by hijacking the host E3 ligase complex, and we propose the novel molecular mechanism of telomerase deregulation in possibly HIV-1 pathogenesis.
Keywords: DYRK2; EDD; HIV-1; Protein Degradation; TERT; Telomerase; Telomeres; Ubiquitination; VPRBP; Vpr.
Figures




References
-
- Margottin F., Bour S. P., Durand H., Selig L., Benichou S., Richard V., Thomas D., Strebel K., Benarous R. (1998) A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 - PubMed
-
- Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X. F. (2003) Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302, 1056–1060 - PubMed
-
- Marin M., Rose K. M., Kozak S. L., Kabat D. (2003) HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9, 1398–1403 - PubMed
-
- Sheehy A. M., Gaddis N. C., Malim M. H. (2003) The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9, 1404–1407 - PubMed
-
- Mariani R., Chen D., Schröfelbauer B., Navarro F., König R., Bollman B., Münk C., Nymark-McMahon H., Landau N. R. (2003) Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114, 21–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

