Mammalian target of rapamycin complex 1 (mTORC1) enhances bortezomib-induced death in tuberous sclerosis complex (TSC)-null cells by a c-MYC-dependent induction of the unfolded protein response
- PMID: 23612979
- PMCID: PMC3668728
- DOI: 10.1074/jbc.M112.431056
Mammalian target of rapamycin complex 1 (mTORC1) enhances bortezomib-induced death in tuberous sclerosis complex (TSC)-null cells by a c-MYC-dependent induction of the unfolded protein response
Abstract
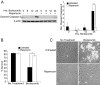
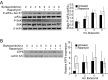
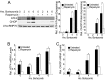
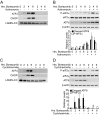
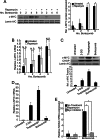
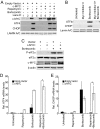
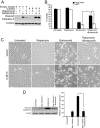
Many factors, including duration and intensity of the unfolded protein response (UPR), dictate whether cells will adapt to endoplasmic reticulum stress or undergo apoptosis. In tuberous sclerosis (TSC), elevation of mammalian target of rapamycin complex 1 (mTORC1) activity has been proposed to compound the induction of UPR transcription factors ATF4 and CHOP, suggesting that the UPR could be targeted to eradicate TSC1/2-null cells during patient therapy. Here we report that control of c-MYC translation by mTORC1 plays a key role in determining whether TSC2-null Elt3 rat leiomyoma cells apoptose in response to UPR induction by the proteasome inhibitor bortezomib. Although bortezomib induces eukaryotic initiating factor 2α phosphorylation, mTORC1 activity was also required for downstream induction of the UPR transcription factors ATF4 and CHOP by a mechanism involving increased expression of c-MYC. Although bortezomib-induced c-MYC transcription was resistant to rapamycin treatment, mTORC1 activity was required for efficient c-MYC translation. c-MYC subsequently bound to the ATF4 promoter, suggesting direct involvement of an mTORC1/c-MYC-driven signaling pathway in the activation of the UPR. Consistent with this notion, exogenously expressed c-MYC reversed the ability of rapamycin to prevent bortezomib-induced CHOP and ATF4 expression as well as apoptosis. These findings indicate that the induction of ATF4/CHOP expression occurs via mTORC1 regulation of c-MYC and that this signaling pathway is a major determinant in the ability of bortezomib to induce apoptosis.
Keywords: ATF4; Apoptosis; Bortezomib; Myc; Rapamycin; Tuberous Sclerosis (Tsc); Unfolded Protein Response; mTOR.
Figures

References
-
- Crino P. B., Nathanson K. L., Henske E. P. (2006) The tuberous sclerosis complex. New Eng. J. Med. 355, 1345–1356 - PubMed
-
- Castro A. F., Rebhun J. F., Clark G. J., Quilliam L. A. (2003) Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J. Biol. Chem. 278, 32493–32496 - PubMed
-
- Zhang Y., Gao X., Saucedo L. J., Ru B., Edgar B. A., Pan D. (2003) Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 5, 578–581 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

