BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis
- PMID: 23613197
- PMCID: PMC3663264
- DOI: 10.1105/tpc.112.109090
BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis
Abstract
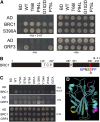
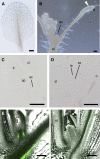
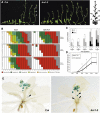
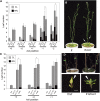
Plant architecture shows a large degree of developmental plasticity. Some of the key determinants are the timing of the floral transition induced by a systemic flowering signal (florigen) and the branching pattern regulated by key factors such as BRANCHED1 (BRC1). Here, we report that BRC1 interacts with the florigen proteins FLOWERING LOCUS T (FT) and TWIN SISTER OF FT (TSF) but not with TERMINAL FLOWER1, a floral repressor. FT protein induced in leaves moves into the subtended bud, suggesting that FT protein also plays a role in promotion of the floral transition in the axillary meristem (AM). The brc1-2 mutant shows an earlier floral transition in the axillary shoots compared with the wild type, suggesting that BRC1 plays a role in delaying the floral transition of the AMs. Genetic and gene expression analyses suggest that BRC1 interferes with florigen (FT and TSF) function in the AMs. Consistent with this, BRC1 ectopically expressed in the shoot apical meristem delays the floral transition in the main shoot. These results taken together suggest that BRC1 protein interacts with FT and TSF proteins and modulates florigen activity in the axillary buds to prevent premature floral transition of the AMs.
Figures

References
-
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 - PubMed
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Alvarez J., Guli C., Yu X., Smyth D. (1992). terminal flower: A gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 2: 103–116
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

