Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages
- PMID: 23613317
- PMCID: PMC3742575
- DOI: 10.1158/1078-0432.CCR-12-3314
Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages
Abstract
Purpose: Gliomas are known to induce local and systemic immunosuppression, inhibiting T-cell-mediated cytotoxic responses to tumor growth. Tumor-associated macrophages are a significant component of the immune infiltrate in gliomas and may express immunosuppressive surface ligands, such as B7-H1.
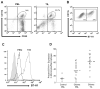
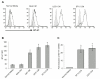
Experimental design: Tumor and peripheral blood samples from patients with glioblastoma (GBM) were analyzed by flow cytometry to evaluate the expression of B7-H1 in circulating and tumor-infiltrating macrophages. Human monocytes from healthy patients were stimulated with conditioned media from glioma cells to evaluate B7-H1 expression. Production of interleukin (IL)-10 by stimulated monocytes was measured by ELISA, and stimulation with IL-10 alone was evaluated for the ability to induce B7-H1 expression. The effect of inhibiting IL-10 and its receptor on glioma-induced B7-H1 expression in monocytes was evaluated.
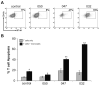
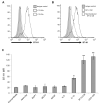
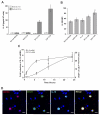
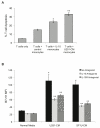
Results: Circulating monocytes in patients with GBM had significantly increased expression of B7-H1 compared with healthy control patients. Tumor-associated macrophages from matched GBM tissue had even greater B7-H1 expression. Treatment of normal monocytes with glioma-conditioned media could significantly increase B7-H1 expression. Stimulation of monocytes with conditioned media resulted in substantial production of IL-10 and upregulation of the IL-10 receptor. Stimulation of monocytes with IL-10 alone could significantly increase B7-H1 expression, sufficient to induce T-cell apoptosis when cocultured with stimulated monocytes. Inhibition of IL-10 and the IL-10 receptor could knock down the effect of glioma media on B7-H1 by more than 50%.
Conclusions: Gliomas can upregulate B7-H1 expression in circulating monocytes and tumor-infiltrative macrophages through modulation of autocrine/paracrine IL-10 signaling, resulting in an immunosuppressive phenotype.
Figures
References
-
- Surawicz TS, Davis F, Freels S, Laws ER, Jr., Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–60. - PubMed
-
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. - PubMed
-
- Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–73. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Gousias K, Markou M, Arzoglou V, Voulgaris S, Vartholomatos G, Kostoula A, et al. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol. 2010;226:136–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

