Social regulation of male reproductive plasticity in an African cichlid fish
- PMID: 23613320
- PMCID: PMC3836007
- DOI: 10.1093/icb/ict017
Social regulation of male reproductive plasticity in an African cichlid fish
Abstract
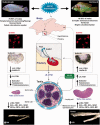
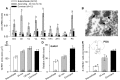
Social interactions with the outcome of a position in a dominance hierarchy can have profound effects on reproductive behavior and physiology, requiring animals to integrate environmental information with their internal physiological state; but how is salient information from the animal's dynamic social environment transformed into adaptive behavioral, physiological, and molecular-level changes? The African cichlid fish, Astatotilapia burtoni, is ideally suited to understand socially controlled reproductive plasticity because activity of the male reproductive (brain-pituitary-gonad) axis is tightly linked to social status. Males form hierarchies in which a small percentage of brightly colored dominant individuals have an active reproductive axis, defend territories, and spawn with females, while the remaining males are subordinate, drably colored, do not hold a territory, and have a suppressed reproductive system with minimal opportunities for spawning. These social phenotypes are plastic and quickly reversible, meaning that individual males may switch between dominant and subordinate status multiple times within a lifetime. Here, we review the rapid and remarkable plasticity that occurs along the entire reproductive axis when males rise in social rank, a transition that has important implications for the operational sex ratio of the population. When males rise in rank, transformations occur in the brain, pituitary, circulation, and testes over short time-scales (minutes to days). Changes are evident in overt behavior, as well as modifications at the physiological, cellular, and molecular levels that regulate reproductive capacity. Widespread changes triggered by a switch in rank highlight the significance of external social information in shaping internal physiology and reproductive competence.
Figures
Similar articles
-
Social Transitions Cause Rapid Behavioral and Neuroendocrine Changes.Integr Comp Biol. 2015 Aug;55(2):294-306. doi: 10.1093/icb/icv057. Epub 2015 Jun 1. Integr Comp Biol. 2015. PMID: 26037297 Free PMC article. Review.
-
Plasticity of the reproductive axis caused by social status change in an african cichlid fish: I. Pituitary gonadotropins.Endocrinology. 2011 Jan;152(1):281-90. doi: 10.1210/en.2010-0875. Epub 2010 Nov 10. Endocrinology. 2011. PMID: 21068157 Free PMC article.
-
Social regulation of reproduction in male cichlid fishes.Gen Comp Endocrinol. 2014 Oct 1;207:2-12. doi: 10.1016/j.ygcen.2014.04.038. Epub 2014 May 20. Gen Comp Endocrinol. 2014. PMID: 24859257 Review.
-
Physiological consequences of social descent: studies in Astatotilapia burtoni.J Endocrinol. 2006 Jul;190(1):183-90. doi: 10.1677/joe.1.06755. J Endocrinol. 2006. PMID: 16837622
-
Plasticity of the reproductive axis caused by social status change in an african cichlid fish: II. testicular gene expression and spermatogenesis.Endocrinology. 2011 Jan;152(1):291-302. doi: 10.1210/en.2010-0876. Epub 2010 Nov 17. Endocrinology. 2011. PMID: 21084443 Free PMC article.
Cited by
-
Evolutionary diversity as a catalyst for biological discovery.Integr Zool. 2018 Nov;13(6):616-633. doi: 10.1111/1749-4877.12339. Integr Zool. 2018. PMID: 29851228 Free PMC article. Review.
-
Adaptive explanations for sensitive windows in development.Front Zool. 2015 Aug 24;12 Suppl 1(Suppl 1):S3. doi: 10.1186/1742-9994-12-S1-S3. eCollection 2015. Front Zool. 2015. PMID: 26816521 Free PMC article. Review.
-
Adaptive reshaping of the hormonal phenotype after social niche transition in adulthood.Proc Biol Sci. 2020 Jun 10;287(1928):20200667. doi: 10.1098/rspb.2020.0667. Epub 2020 Jun 10. Proc Biol Sci. 2020. PMID: 32517608 Free PMC article.
-
Brain transcriptomics of agonistic behaviour in the weakly electric fish Gymnotus omarorum, a wild teleost model of non-breeding aggression.Sci Rep. 2020 Jun 11;10(1):9496. doi: 10.1038/s41598-020-66494-9. Sci Rep. 2020. PMID: 32528029 Free PMC article.
-
Dissecting the Transcriptional Patterns of Social Dominance across Teleosts.Integr Comp Biol. 2016 Dec;56(6):1250-1265. doi: 10.1093/icb/icw118. Integr Comp Biol. 2016. PMID: 27940616 Free PMC article.
References
-
- Alonso F, Honji RM, Guimaraes Moreira R, Pandolfi M. Dominance hierarchies and social status ascent opportunity: anticipatory behavioral and physiological adjustments in a Neotropical cichlid fish. Physiol Behav. 2012;106:612–8. - PubMed
-
- Altmann J, Sapolsky R, Licht P. Baboon fertility and social status. Nature. 1995;377:688–90. - PubMed
-
- Au TM, Greenwood AK, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behav Brain Res. 2006;170:342–6. - PubMed
-
- Bass AH, Grober MS. Social and neural modulation of sexual plasticity in teleost fish. Brain Behav Evol. 2001;57:293–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

