Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation
- PMID: 23613521
- PMCID: PMC3674666
- DOI: 10.1182/blood-2013-01-480228
Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation
Abstract
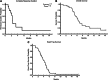
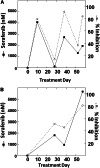
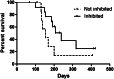
Patients received 5-azacytidine (AZA) 75 mg/m(2) intravenously daily for 7 days and sorafenib 400 mg orally twice daily continuously; cycles were repeated at ~1-month intervals. Forty-three acute myeloid leukemia (AML) patients with a median age of 64 years (range, 24-87 years) were enrolled; 37 were evaluable for response. FMS-like tyrosine kinase-3 (FLT3)-internal tandem duplication (ITD) mutation was detected in 40 (93%) patients, with a median allelic ratio of 0.32 (range, 0.009-0.93). They had received a median of 2 prior treatment regimens (range, 0-7); 9 had failed prior therapy with a FLT3 kinase inhibitor. The response rate was 46%, including 10 (27%) complete response with incomplete count recovery (CRi), 6 (16%) complete responses (CR), and 1 (3%) partial response. The median time to achieve CR/CRi was 2 cycles (range, 1-4), and the median duration of CR/CRi was 2.3 months (range, 1-14.3 months). Sixty-four percent of patients achieved adequate (defined as >85%) FLT3 inhibition during their first cycle of therapy. The degree of FLT3 inhibition correlated with plasma sorafenib concentrations. FLT3 ligand levels did not rise to levels seen in prior studies of patients receiving cytotoxic chemotherapy. The combination of AZA and sorafenib is effective for patients with relapsed AML and FLT-3-ITD. This trial was registered at clinicaltrials.gov as #NCT01254890.
Figures


References
-
- Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. - PubMed
-
- Fröhling S, Schlenk RF, Breitruck J, et al. AML Study Group Ulm. Acute myeloid leukemia. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. - PubMed
-
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. - PubMed
-
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111(1):190–195. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

