Organization and function of transmitter release sites at the neuromuscular junction
- PMID: 23613535
- PMCID: PMC3717219
- DOI: 10.1113/jphysiol.2012.248625
Organization and function of transmitter release sites at the neuromuscular junction
Abstract
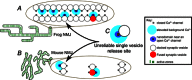
The neuromuscular junction is known as a strong and reliable synapse. It is strong because it releases an excess of chemical transmitter, beyond what is required to bring the postsynaptic muscle cell to threshold. Because the synapse can sustain suprathreshold muscle activation during short trains of action potentials, it is also reliable. The presynaptic mechanisms that lead to reliability during short trains of activity have only recently been elucidated. It appears that there are relatively few calcium channels in individual active zones, that channels open with a low probability during action potential stimulation and that even if channels open the resulting calcium flux only rarely triggers vesicle fusion. Thus, each synaptic vesicle may only associate with a small number of calcium channels, forming an unreliable single vesicle release site. Strength and reliability of the neuromuscular junction emerge as a result of its assembly from thousands of these unreliable single vesicle release sites. Hence, these synapses are strong while at the same time only releasing a small subset of available docked vesicles during each action potential, thus conserving transmitter release resources. This prevents significant depression during short trains of action potential activity and confers reliability.
Figures


References
-
- Bekkers JM. Quantal analysis of synaptic transmission in the central nervous system. Curr Opin Neurobiol. 1994;4:360–365. - PubMed
-
- Bennett MR. Neuromuscular transmission at an active zone: the secretosome hypothesis. J Neurocytol. 1996;25:869–891. - PubMed
-
- Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis. Nat Rev Mol Cell Biol. 2002;3:498–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

