Noninvasive prenatal molecular karyotyping from maternal plasma
- PMID: 23613765
- PMCID: PMC3629174
- DOI: 10.1371/journal.pone.0060968
Noninvasive prenatal molecular karyotyping from maternal plasma
Abstract
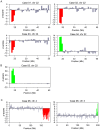
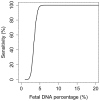
Fetal DNA is present in the plasma of pregnant women. Massively parallel sequencing of maternal plasma DNA has been used to detect fetal trisomies 21, 18, 13 and selected sex chromosomal aneuploidies noninvasively. Case reports describing the detection of fetal microdeletions from maternal plasma using massively parallel sequencing have been reported. However, these previous reports were either polymorphism-dependent or used statistical analyses which were confined to one or a small number of selected parts of the genome. In this report, we reported a procedure for performing noninvasive prenatal karyotyping at 3 Mb resolution across the whole genome through the massively parallel sequencing of maternal plasma DNA. This method has been used to analyze the plasma obtained from 6 cases. In three cases, fetal microdeletions have been detected successfully from maternal plasma. In two cases, fetal microduplications have been detected successfully from maternal plasma. In the remaining case, the plasma DNA sequencing result was consistent with the pregnant mother being a carrier of a microduplication. Simulation analyses were performed for determining the number of plasma DNA molecules that would need to be sequenced and aligned for enhancing the diagnostic resolution of noninvasive prenatal karyotyping to 2 Mb and 1 Mb. In conclusion, noninvasive prenatal molecular karyotyping from maternal plasma by massively parallel sequencing is feasible and would enhance the diagnostic spectrum of noninvasive prenatal testing.
Conflict of interest statement
Figures

References
-
- Lo YMD, Corbetta N, Chamberlain PF, Rai V, Sargent IL, et al. (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350: 485–487. - PubMed
-
- Lo YMD, Chiu RWK (2012) Genomic analysis of fetal nucleic acids in maternal blood. Annu Rev Genomics Hum Genet 13: 285–306. - PubMed
-
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE, Neveux LM, et al. (2011) DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med 13: 913–920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

