Superoxide mediates direct current electric field-induced directional migration of glioma cells through the activation of AKT and ERK
- PMID: 23613809
- PMCID: PMC3629049
- DOI: 10.1371/journal.pone.0061195
Superoxide mediates direct current electric field-induced directional migration of glioma cells through the activation of AKT and ERK
Abstract
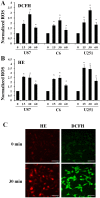
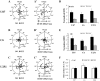
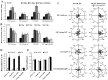
Direct current electric fields (DCEFs) can induce directional migration for many cell types through activation of intracellular signaling pathways. However, the mechanisms that bridge extracellular electrical stimulation with intracellular signaling remain largely unknown. In the current study, we found that a DCEF can induce the directional migration of U87, C6 and U251 glioma cells to the cathode and stimulate the production of hydrogen peroxide and superoxide. Subsequent studies demonstrated that the electrotaxis of glioma cells were abolished by the superoxide inhibitor N-acetyl-l-cysteine (NAC) or overexpression of mitochondrial superoxide dismutase (MnSOD), but was not affected by inhibition of hydrogen peroxide through the overexpression of catalase. Furthermore, we found that the presence of NAC, as well as the overexpression of MnSOD, could almost completely abolish the activation of Akt, extracellular-signal-regulated kinase (Erk)1/2, c-Jun N-terminal kinase (JNK), and p38, although only JNK and p38 were affected by overexpression of catalase. The presenting of specific inhibitors can decrease the activation of Erk1/2 or Akt as well as the directional migration of glioma cells. Collectively, our data demonstrate that superoxide may play a critical role in DCEF-induced directional migration of glioma cells through the regulation of Akt and Erk1/2 activation. This study provides novel evidence that the superoxide is at least one of the "bridges" coupling the extracellular electric stimulation to the intracellular signals during DCEF-mediated cell directional migration.
Conflict of interest statement
Figures




Similar articles
-
Superoxide plays critical roles in electrotaxis of fibrosarcoma cells via activation of ERK and reorganization of the cytoskeleton.Free Radic Biol Med. 2012 May 1;52(9):1888-96. doi: 10.1016/j.freeradbiomed.2012.02.047. Epub 2012 Mar 8. Free Radic Biol Med. 2012. PMID: 22406317
-
Hydrogen peroxide contributes to the manganese superoxide dismutase promotion of migration and invasion in glioma cells.Free Radic Res. 2011 Oct;45(10):1154-61. doi: 10.3109/10715762.2011.604321. Epub 2011 Aug 5. Free Radic Res. 2011. PMID: 21749277
-
Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides.Neuro Oncol. 2011 Sep;13(9):961-73. doi: 10.1093/neuonc/nor068. Epub 2011 Jun 22. Neuro Oncol. 2011. PMID: 21697181 Free PMC article.
-
c-jun amino-terminal kinase and mitogen activated protein kinase 1/2 mediate hepatocyte growth factor-induced migration of brain endothelial cells.Exp Cell Res. 2007 Jan 1;313(1):121-32. doi: 10.1016/j.yexcr.2006.09.018. Epub 2006 Sep 26. Exp Cell Res. 2007. PMID: 17055484
-
Recent Developments in Electrotaxis Assays.Adv Wound Care (New Rochelle). 2014 Feb 1;3(2):149-155. doi: 10.1089/wound.2013.0453. Adv Wound Care (New Rochelle). 2014. PMID: 24761355 Free PMC article. Review.
Cited by
-
Migration of Human Renal Tubular Epithelial Cells in Response to Physiological Electric Signals.Front Cell Dev Biol. 2021 Sep 14;9:724012. doi: 10.3389/fcell.2021.724012. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34595174 Free PMC article.
-
Galvanotactic Migration of Glioblastoma and Brain Metastases Cells.Life (Basel). 2022 Apr 14;12(4):580. doi: 10.3390/life12040580. Life (Basel). 2022. PMID: 35455071 Free PMC article.
-
Pulsed DC Electric Field-Induced Differentiation of Cortical Neural Precursor Cells.PLoS One. 2016 Jun 28;11(6):e0158133. doi: 10.1371/journal.pone.0158133. eCollection 2016. PLoS One. 2016. PMID: 27352251 Free PMC article.
-
The Galvanotactic Migration of Keratinocytes is Enhanced by Hypoxic Preconditioning.Sci Rep. 2015 May 19;5:10289. doi: 10.1038/srep10289. Sci Rep. 2015. PMID: 25988491 Free PMC article.
-
Glioblastoma U-87 cell electrotaxis is hindered by doxycycline with a concomitant reduction in the matrix metallopeptidase-9 expression.Biochem Biophys Rep. 2024 Mar 25;38:101690. doi: 10.1016/j.bbrep.2024.101690. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38571555 Free PMC article.
References
-
- Sato M, Ueda M, Takagi H, Watanabe T, Yanagida T (2007) Input–output relationship in galvanotactic response of Dictyostelium cells. Biosystems 88: 261–272. - PubMed
-
- Li J, Nandagopal S, Wu D, Romanuik SF, Paul K, et al. (2011) Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 11: 1298–1304. - PubMed
-
- Zhao M (2009) Electrical fields in wound healing-An overriding signal that directs cell migration. Seminars in Cell & Developmental Biology 20: 674–682. - PubMed
-
- Yan X, Han J, Zhang Z, Wang J, Cheng Q, et al. (2009) Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics 30: 29–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

