AMIGO3 is an NgR1/p75 co-receptor signalling axon growth inhibition in the acute phase of adult central nervous system injury
- PMID: 23613963
- PMCID: PMC3628841
- DOI: 10.1371/journal.pone.0061878
AMIGO3 is an NgR1/p75 co-receptor signalling axon growth inhibition in the acute phase of adult central nervous system injury
Abstract
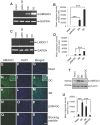
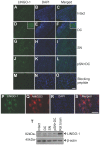
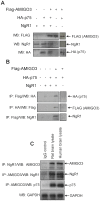
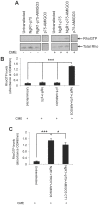
Axon regeneration in the injured adult CNS is reportedly inhibited by myelin-derived inhibitory molecules, after binding to a receptor complex comprised of the Nogo-66 receptor (NgR1) and two transmembrane co-receptors p75/TROY and LINGO-1. However, the post-injury expression pattern for LINGO-1 is inconsistent with its proposed function. We demonstrated that AMIGO3 levels were significantly higher acutely than those of LINGO-1 in dorsal column lesions and reduced in models of dorsal root ganglion neuron (DRGN) axon regeneration. Similarly, AMIGO3 levels were raised in the retina immediately after optic nerve crush, whilst levels were suppressed in regenerating optic nerves, induced by intravitreal peripheral nerve implantation. AMIGO3 interacted functionally with NgR1-p75/TROY in non-neuronal cells and in brain lysates, mediating RhoA activation in response to CNS myelin. Knockdown of AMIGO3 in myelin-inhibited adult primary DRG and retinal cultures promoted disinhibited neurite growth when cells were stimulated with appropriate neurotrophic factors. These findings demonstrate that AMIGO3 substitutes for LINGO-1 in the NgR1-p75/TROY inhibitory signalling complex and suggests that the NgR1-p75/TROY-AMIGO3 receptor complex mediates myelin-induced inhibition of axon growth acutely in the CNS. Thus, antagonizing AMIGO3 rather than LINGO-1 immediately after CNS injury is likely to be a more effective therapeutic strategy for promoting CNS axon regeneration when combined with neurotrophic factor administration.
Conflict of interest statement
Figures




References
-
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A (2004) Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia 46: 225–251. - PubMed
-
- Hunt D, Coffin RS, Anderson PN (2002) The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review. J Neurocytol 31: 93–120. - PubMed
-
- Berry M, Ahmed Z, Lorber B, Douglas M, Logan A (2008) Regeneration of axons in the visual system. Restor Neurol Neurosci 26: 147–174. - PubMed
-
- Filbin MT (2003) Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 4: 703–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

