Paraquat modulates alternative pre-mRNA splicing by modifying the intracellular distribution of SRPK2
- PMID: 23613995
- PMCID: PMC3628584
- DOI: 10.1371/journal.pone.0061980
Paraquat modulates alternative pre-mRNA splicing by modifying the intracellular distribution of SRPK2
Abstract
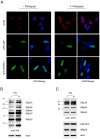
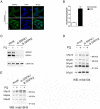
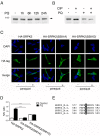
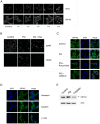
Paraquat (PQ) is a neurotoxic herbicide that induces superoxide formation. Although it is known that its toxic properties are linked to ROS production, the cellular response to PQ is still poorly understood. We reported previously that treatment with PQ induced genome-wide changes in pre-mRNA splicing. Here, we investigated the molecular mechanism underlying PQ-induced pre-mRNA splicing alterations. We show that PQ treatment leads to the phosphorylation and nuclear accumulation of SRPK2, a member of the family of serine/arginine (SR) protein-specific kinases. Concomitantly, we observed increased phosphorylation of SR proteins. Site-specific mutagenesis identified a single serine residue that is necessary and sufficient for nuclear localization of SRPK2. Transfection of a phosphomimetic mutant modified splice site selection of the E1A minigene splicing reporter similar to PQ-treatment. Finally, we found that PQ induces DNA damage and vice versa that genotoxic treatments are also able to promote SRPK2 phosphorylation and nuclear localization. Consistent with these observations, treatment with PQ, cisplatin or γ-radiation promote changes in the splicing pattern of genes involved in DNA repair, cell cycle control, and apoptosis. Altogether, our findings reveal a novel regulatory mechanism that connects PQ to the DNA damage response and to the modulation of alternative splicing via SRPK2 phosphorylation.
Conflict of interest statement
Figures


Similar articles
-
SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells.J Cell Biol. 1998 Feb 23;140(4):737-50. doi: 10.1083/jcb.140.4.737. J Cell Biol. 1998. PMID: 9472028 Free PMC article.
-
Increased Serine-Arginine (SR) Protein Phosphorylation Changes Pre-mRNA Splicing in Hypoxia.J Biol Chem. 2015 Jul 17;290(29):18079-18089. doi: 10.1074/jbc.M115.639690. Epub 2015 May 28. J Biol Chem. 2015. PMID: 26023237 Free PMC article.
-
Inhibitors of CLK protein kinases suppress cell growth and induce apoptosis by modulating pre-mRNA splicing.PLoS One. 2015 Jan 12;10(1):e0116929. doi: 10.1371/journal.pone.0116929. eCollection 2015. PLoS One. 2015. PMID: 25581376 Free PMC article.
-
Connecting the speckles: Splicing kinases and their role in tumorigenesis and treatment response.Nucleus. 2015;6(4):279-88. doi: 10.1080/19491034.2015.1062194. Epub 2015 Jun 22. Nucleus. 2015. PMID: 26098145 Free PMC article. Review.
-
SR protein kinases: the splice of life.Biochem Cell Biol. 1999;77(4):293-8. Biochem Cell Biol. 1999. PMID: 10546892 Review.
Cited by
-
Good Cop, Bad Cop: The Different Roles of SRPKs.Front Genet. 2022 Jun 2;13:902718. doi: 10.3389/fgene.2022.902718. eCollection 2022. Front Genet. 2022. PMID: 35719374 Free PMC article. Review.
-
Nuclear Translocation of SRPKs Is Associated with 5-FU and Cisplatin Sensitivity in HeLa and T24 Cells.Cells. 2021 Mar 30;10(4):759. doi: 10.3390/cells10040759. Cells. 2021. PMID: 33808326 Free PMC article.
-
Transcriptomic variation of pharmacogenes in multiple human tissues and lymphoblastoid cell lines.Pharmacogenomics J. 2017 Mar;17(2):137-145. doi: 10.1038/tpj.2015.93. Epub 2016 Feb 9. Pharmacogenomics J. 2017. PMID: 26856248 Free PMC article.
-
Global variability in gene expression and alternative splicing is modulated by mitochondrial content.Genome Res. 2015 May;25(5):633-44. doi: 10.1101/gr.178426.114. Epub 2015 Mar 23. Genome Res. 2015. PMID: 25800673 Free PMC article.
-
Genome-scale analysis of Arabidopsis splicing-related protein kinase families reveals roles in abiotic stress adaptation.BMC Plant Biol. 2022 Oct 22;22(1):496. doi: 10.1186/s12870-022-03870-9. BMC Plant Biol. 2022. PMID: 36273172 Free PMC article.
References
-
- Cocheme HM, Murphy MP (2008) Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283: 1786–1798. - PubMed
-
- Lenzken SC, Romeo V, Zolezzi F, Cordero F, Lamorte G, et al. (2011) Mutant SOD1 and mitochondrial damage alter expression and splicing of genes controlling neuritogenesis in models of neurodegeneration. Hum Mutat 32: 168–182. - PubMed
-
- Schwerk C, Schulze-Osthoff K (2005) Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell 19: 1–13. - PubMed
-
- Shin C, Manley JL (2004) Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol 5: 727–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

