Metabolism of 2-chloro-4-nitroaniline via novel aerobic degradation pathway by Rhodococcus sp. strain MB-P1
- PMID: 23614030
- PMCID: PMC3629101
- DOI: 10.1371/journal.pone.0062178
Metabolism of 2-chloro-4-nitroaniline via novel aerobic degradation pathway by Rhodococcus sp. strain MB-P1
Retraction in
-
Retraction: Metabolism of 2-chloro-4-nitroaniline via novel aerobic degradation pathway by Rhodococcus sp. strain MB-P1.PLoS One. 2014 Jul 9;9(7):e102862. doi: 10.1371/journal.pone.0102862. eCollection 2014. PLoS One. 2014. PMID: 25006667 Free PMC article. No abstract available.
Abstract
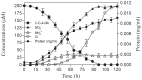
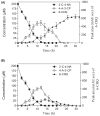
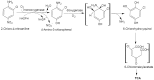
2-chloro-4-nitroaniline (2-C-4-NA) is used as an intermediate in the manufacture of dyes, pharmaceuticals, corrosion inhibitor and also used in the synthesis of niclosamide, a molluscicide. It is marked as a black-listed substance due to its poor biodegradability. We report biodegradation of 2-C-4-NA and its pathway characterization by Rhodococcus sp. strain MB-P1 under aerobic conditions. The strain MB-P1 utilizes 2-C-4-NA as the sole carbon, nitrogen, and energy source. In the growth medium, the degradation of 2-C-4-NA occurs with the release of nitrite ions, chloride ions, and ammonia. During the resting cell studies, the 2-C-4-NA-induced cells of strain MB-P1 transformed 2-C-4-NA stoichiometrically to 4-amino-3-chlorophenol (4-A-3-CP), which subsequently gets transformed to 6-chlorohydroxyquinol (6-CHQ) metabolite. Enzyme assays by cell-free lysates prepared from 2-C-4-NA-induced MB-P1 cells, demonstrated that the first enzyme in the 2-C-4-NA degradation pathway is a flavin-dependent monooxygenase that catalyzes the stoichiometric removal of nitro group and production of 4-A-3-CP. Oxygen uptake studies on 4-A-3-CP and related anilines by 2-C-4-NA-induced MB-P1 cells demonstrated the involvement of aniline dioxygenase in the second step of 2-C-4-NA degradation. This is the first report showing 2-C-4-NA degradation and elucidation of corresponding metabolic pathway by an aerobic bacterium.
Conflict of interest statement
Figures
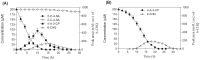

Similar articles
-
Aerobic degradation of N-methyl-4-nitroaniline (MNA) by Pseudomonas sp. strain FK357 isolated from soil.PLoS One. 2013 Oct 8;8(10):e75046. doi: 10.1371/journal.pone.0075046. eCollection 2013. PLoS One. 2013. Retraction in: PLoS One. 2014 Jul 09;9(7):e102855. doi: 10.1371/journal.pone.0102855. PMID: 24116023 Free PMC article. Retracted.
-
RETRACTED: Aerobic degradation of 4-nitroaniline (4-NA) via novel degradation intermediates by Rhodococcus sp. strain FK48.J Hazard Mater. 2013 Jun 15;254-255:72-78. doi: 10.1016/j.jhazmat.2013.03.021. Epub 2013 Mar 16. J Hazard Mater. 2013. Retraction in: J Hazard Mater. 2017 Oct 15;340:486. doi: 10.1016/j.jhazmat.2016.08.062. PMID: 23587930 Retracted.
-
Novel catabolic pathway for 4-Nitroaniline in a Rhodococcus sp. strain JS360.J Hazard Mater. 2023 Jul 15;454:131473. doi: 10.1016/j.jhazmat.2023.131473. Epub 2023 Apr 23. J Hazard Mater. 2023. PMID: 37146325
-
Isolation and characterization of an atrazine-degrading Rhodococcus sp. strain MB-P1 from contaminated soil.Lett Appl Microbiol. 2009 Dec;49(6):721-9. doi: 10.1111/j.1472-765X.2009.02724.x. Epub 2009 Aug 22. Lett Appl Microbiol. 2009. PMID: 19818008
-
Degradation of 2-methylaniline and chlorinated isomers of 2-methylaniline by Rhodococcus rhodochrous strain CTM.J Gen Microbiol. 1991 Aug;137(8):2033-9. doi: 10.1099/00221287-137-8-2033. J Gen Microbiol. 1991. PMID: 1955877
Cited by
-
Retraction: Metabolism of 2-chloro-4-nitroaniline via novel aerobic degradation pathway by Rhodococcus sp. strain MB-P1.PLoS One. 2014 Jul 9;9(7):e102862. doi: 10.1371/journal.pone.0102862. eCollection 2014. PLoS One. 2014. PMID: 25006667 Free PMC article. No abstract available.
-
Biodegradation of the allelopathic chemical m-tyrosine by Bacillus aquimaris SSC5 involves the homogentisate central pathway.PLoS One. 2013 Oct 1;8(10):e75928. doi: 10.1371/journal.pone.0075928. eCollection 2013. PLoS One. 2013. Retraction in: PLoS One. 2014 Jul 09;9(7):e102854. doi: 10.1371/journal.pone.0102854. PMID: 24098407 Free PMC article. Retracted.
-
Aerobic degradation of N-methyl-4-nitroaniline (MNA) by Pseudomonas sp. strain FK357 isolated from soil.PLoS One. 2013 Oct 8;8(10):e75046. doi: 10.1371/journal.pone.0075046. eCollection 2013. PLoS One. 2013. Retraction in: PLoS One. 2014 Jul 09;9(7):e102855. doi: 10.1371/journal.pone.0102855. PMID: 24116023 Free PMC article. Retracted.
References
-
- Hampercht R, Westerkamp A (2000) Disperse Dyes. Ullmann's Encylopedia of Industrial Chemistry (7th Edition). NY, NY: John Wiley and Sons. Published online: 15 Sept 2000.
-
- Lewis RJ, Sr. Hawley's Condensed Chemical Dictionary (14th Edition) (2002). John Wiley and Sons, Inc. New York, NY P. 255.
-
- Schnorbach HJ, Matthaei HD, Muller F (2008) Molluskicides. Ullmann's Encylopedia of Industrial Chemistry (7th Edition). NY, John Wiley and Sons. Published online: 15 Oct 2008.
-
- Graebing PW, Chib JS, Hubert TD, Gingerich WH (2004) Aqueous photolysis of niclosamide. J Agric Food Chem 52: 870–878. - PubMed
-
- Espinosa-Aquirre JJ, Reyes RE, Cortinas de Nava C (1991) Mutagenic activity of 2-chloro-4-nitroaniline and 5-chlorosalicyclic acid in Salmonella typhimurium: two possible metabolites of niclosamide. Mutat Res 264: 139–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

