Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes
- PMID: 23614626
- PMCID: PMC3775905
- DOI: 10.1021/bi301657w
Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes
Abstract
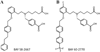
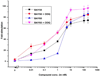
The soluble guanylyl cyclase (sGC) is an important receptor for nitric oxide (NO). Nitric oxide activates sGC several hundred fold to generate cGMP from GTP. Because of sGC's salutary roles in cardiovascular physiology, it has received substantial attention as a drug target. The heme domain of sGC is key to its regulation as it not only contains the NO activation site but also harbors sites for NO-independent sGC activators as well an S-nitrosylation site (β1 C122) involved in desensitization. Here we report the crystal structure of the activator BAY 60-2770 bound to the Nostoc H-NOX domain that is homologous to sGC. The structure reveals that BAY 60-2770 has displaced the heme and acts as a heme mimetic via carboxylate-mediated interactions with the conserved YxSxR motif as well as hydrophobic interactions. Comparisons with the previously determined BAY 58-2667 bound structure reveal that BAY 60-2770 is more ordered in its hydrophobic tail region. sGC activity assays demonstrate that BAY 60-2770 has about 10% higher fold maximal stimulation compared to BAY 58-2667. S-Nitrosylation of the BAY 60-2770 substituted Nostoc H-NOX domain causes subtle changes in the vicinity of the S-nitrosylated C122 residue. These shifts could impact the adjacent YxSxR motif and αF helix and as such potentially inhibit either heme incorporation or NO-activation of sGC and thus provide a structural basis for desensitization.
Figures
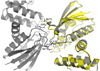
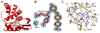


Similar articles
-
Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase.J Biol Chem. 2010 Jul 16;285(29):22651-7. doi: 10.1074/jbc.M110.111559. Epub 2010 May 12. J Biol Chem. 2010. PMID: 20463019 Free PMC article.
-
Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation.Proc Natl Acad Sci U S A. 2007 Jul 24;104(30):12312-7. doi: 10.1073/pnas.0703944104. Epub 2007 Jul 16. Proc Natl Acad Sci U S A. 2007. PMID: 17636120 Free PMC article.
-
Insights into soluble guanylyl cyclase activation derived from improved heme-mimetics.J Med Chem. 2013 Nov 14;56(21):8948-8952. doi: 10.1021/jm400539d. Epub 2013 Oct 24. J Med Chem. 2013. PMID: 24090476 Free PMC article.
-
How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c', Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase.Antioxid Redox Signal. 2012 Nov 1;17(9):1246-63. doi: 10.1089/ars.2012.4564. Epub 2012 Apr 10. Antioxid Redox Signal. 2012. PMID: 22356101 Free PMC article. Review.
-
Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease.Cardiovasc Drug Rev. 2007 Spring;25(1):30-45. doi: 10.1111/j.1527-3466.2007.00003.x. Cardiovasc Drug Rev. 2007. PMID: 17445086 Review.
Cited by
-
Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):E2355-62. doi: 10.1073/pnas.1524398113. Epub 2016 Apr 11. Proc Natl Acad Sci U S A. 2016. PMID: 27071111 Free PMC article.
-
Maturation, inactivation, and recovery mechanisms of soluble guanylyl cyclase.J Biol Chem. 2021 Jan-Jun;296:100336. doi: 10.1016/j.jbc.2021.100336. Epub 2021 Jan 26. J Biol Chem. 2021. PMID: 33508317 Free PMC article. Review.
-
New insights into the role of soluble guanylate cyclase in blood pressure regulation.Curr Opin Nephrol Hypertens. 2014 Mar;23(2):135-42. doi: 10.1097/01.mnh.0000441048.91041.3a. Curr Opin Nephrol Hypertens. 2014. PMID: 24419369 Free PMC article. Review.
-
Redox Switches Controlling Nitric Oxide Signaling in the Resistance Vasculature and Implications for Blood Pressure Regulation: Mid-Career Award for Research Excellence 2020.Hypertension. 2021 Sep;78(4):912-926. doi: 10.1161/HYPERTENSIONAHA.121.16493. Epub 2021 Aug 23. Hypertension. 2021. PMID: 34420371 Free PMC article. Review.
-
Stimulators and activators of soluble guanylate cyclase for urogenital disorders.Nat Rev Urol. 2018 Jan;15(1):42-54. doi: 10.1038/nrurol.2017.181. Epub 2017 Nov 14. Nat Rev Urol. 2018. PMID: 29133940 Review.
References
-
- Ashman DF, Lipton R, Melicow MM, Rice TD. Isolation of adenosine 3', 5'-monophosphate and guanosine 3', 5'-monophosphate from rat urine. Biochem Biophys Res Commun. 1963;11:330–334. - PubMed
-
- Bohme E, Munske K, Schultz G. [Formation of cyclic guanosine-3',5'-monophosphate in various rat tissues] Naunyn Schmiedebergs Arch Pharmakol. 1969;264:220–221. - PubMed
-
- Cary SP, Winger JA, Derbyshire ER, Marletta MA. Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci. 2006;31:231–239. - PubMed
-
- Chan NL, Rogers PH, Arnone A. Crystal structure of the S-nitroso form of liganded human hemoglobin. Biochemistry. 1998;37:16459–16464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

