Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: studies in submerged and three-dimensional epidermal equivalent models
- PMID: 23614741
- PMCID: PMC3777415
- DOI: 10.1111/exd.12140
Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: studies in submerged and three-dimensional epidermal equivalent models
Abstract
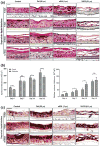
Delphinidin (Del), [3,5,7,3'-,4'-,5'-hexahydroxyflavylium], an anthocyanidin and a potent antioxidant abundantly found in pigmented fruits and vegetables exhibits proapoptotic effects in many cancer cells. Here, we determined the effect of Del on growth, apoptosis and differentiation of normal human epidermal keratinocytes (NHEKs) in vitro in submerged cultures and examined its effects in a three-dimensional (3D) epidermal equivalent (EE) model that permits complete differentiation reminiscent of in vivo skin. Treatment of NHEKs with Del (10-40 μm; 24-48 h) significantly enhanced keratinocyte differentiation. In Del-treated cells, there was marked increase in human involucrin (hINV) promoter activity with simultaneous increase in the mRNA and protein expressions of involucrin and other epidermal differentiation markers including procaspase-14 and transglutaminase-1 (TGM1), but without any effect on TGM2. Del treatment of NHEKs was associated with minimal decrease in cell viability, which was not associated with apoptosis as evident by lack of modulation of caspases, apoptosis-related proteins including Bcl-2 family of proteins and poly(ADP-ribose) polymerase cleavage. To establish the in vivo relevance of our observations in submerged cultures, we then validated these effects in a 3D EE model, where Del was found to significantly enhance cornification and increase the protein expression of cornification markers including caspase-14 and keratin 1. For the first time, we show that Del induces epidermal differentiation using an experimental system that closely mimics in vivo human skin. These observations suggest that Del could be a useful agent for dermatoses associated with epidermal barrier defects including aberrant keratinization, hyperproliferation or inflammation observed in skin diseases like psoriasis and ichthyoses.
© 2013 John Wiley & Sons A/S.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

