Development and mechanism of γ-secretase modulators for Alzheimer's disease
- PMID: 23614767
- PMCID: PMC3796170
- DOI: 10.1021/bi400377p
Development and mechanism of γ-secretase modulators for Alzheimer's disease
Abstract
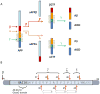
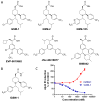
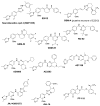
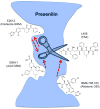
γ-Secretase is an aspartyl intramembranal protease composed of presenilin, Nicastrin, Aph1, and Pen2 with 19 transmembrane domains. γ-Secretase cleaves the amyloid precursor proteins (APP) to release Aβ peptides that likely play a causative role in the pathogenesis of Alzheimer's disease (AD). In addition, γ-secretase cleaves Notch and other type I membrane proteins. γ-Secretase inhibitors (GSIs) have been developed and used for clinical studies. However, clinical trials have shown adverse effects of GSIs that are potentially linked with nondiscriminatory inhibition of Notch signaling, overall APP processing, and other substrate cleavages. Therefore, these findings call for the development of disease-modifying agents that target γ-secretase activity to lower levels of Aβ42 production without blocking the overall processing of γ-secretase substrates. γ-Secretase modulators (GSMs) originally derived from nonsteroidal anti-inflammatory drugs (NSAIDs) display such characteristics and are the focus of this review. However, first-generation GSMs have limited potential because of the low potency and undesired neuropharmacokinetic properties. This generation of GSMs has been suggested to interact with the APP substrate, γ-secretase, or both. To improve the potency and brain availability, second-generation GSMs, including NSAID-derived carboxylic acid and non-NSAID-derived heterocyclic chemotypes, as well as natural product-derived GSMs have been developed. Animal studies of this generation of GSMs have shown encouraging preclinical profiles. Moreover, using potent GSM photoaffinity probes, multiple studies unambiguously have showed that both carboxylic acid and heterocyclic GSMs specifically target presenilin, the catalytic subunit of γ-secretase. In addition, two types of GSMs have distinct binding sites within the γ-secretase complex and exhibit different Aβ profiles. GSMs induce a conformational change of γ-secretase to achieve modulation. Various models are proposed and discussed. Despite the progress of GSM research, many outstanding issues remain to be investigated to achieve the ultimate goal of developing GSMs as effective AD therapies.
Figures


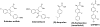


References
-
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. - PubMed
-
- Oehlrich D, Berthelot DJ, Gijsen HJ. gamma-Secretase Modulators as Potential Disease Modifying Anti-Alzheimer’s Drugs. J Med Chem. 2011;54:669–698. - PubMed
-
- Pettersson M, Kauffman GW, am Ende CW, Patel NC, Stiff C, Tran TP, Johnson DS. Novel gamma-secretase modulators: a review of patents from 2008 to 2010. Expert Opin Ther Pat. 2011;21:205–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

