Pd(II)-catalyzed ortho- or meta-C-H olefination of phenol derivatives
- PMID: 23614807
- PMCID: PMC3685289
- DOI: 10.1021/ja400659s
Pd(II)-catalyzed ortho- or meta-C-H olefination of phenol derivatives
Abstract
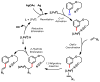
A combination of weakly coordinating auxiliaries and ligand acceleration allows for the development of both ortho- and meta-selective C-H olefination of phenol derivatives. These reactions demonstrate the feasibility of directing C-H functionalizations when functional groups are distal to target C-H bonds. The meta-C-H functionalization of electron-rich phenol derivatives is unprecedented and orthogonal to previous electrophilic substitution of phenols in terms of regioselectivity. These methods are also applied to functionalize α-phenoxyacetic acids, a fibrate class of drug scaffolds.
Figures

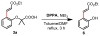
Similar articles
-
Orthogonal selectivity with cinnamic acids in 3-substituted benzofuran synthesis through C-H olefination of phenols.Chem Commun (Camb). 2015 Mar 28;51(25):5375-8. doi: 10.1039/c4cc07026g. Chem Commun (Camb). 2015. PMID: 25347298
-
Pd(II)-catalyzed C-H functionalizations directed by distal weakly coordinating functional groups.J Am Chem Soc. 2015 Apr 8;137(13):4391-7. doi: 10.1021/ja5126897. Epub 2015 Mar 24. J Am Chem Soc. 2015. PMID: 25768039 Free PMC article.
-
Versatile palladium-catalyzed C-H olefination of (hetero)arenes at room temperature.Chem Commun (Camb). 2014 Nov 21;50(90):13914-6. doi: 10.1039/c4cc05827e. Chem Commun (Camb). 2014. PMID: 25260115
-
Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality.Angew Chem Int Ed Engl. 2009;48(28):5094-115. doi: 10.1002/anie.200806273. Angew Chem Int Ed Engl. 2009. PMID: 19557755 Free PMC article. Review.
-
Catalytic synthesis of oligoene and enyne derivatives through carbometalation of internal alkynes.Chem Rec. 2008;8(5):326-36. doi: 10.1002/tcr.20158. Chem Rec. 2008. PMID: 18958852 Review.
Cited by
-
Ligand-Promoted C(sp(3) )-H Olefination en Route to Multi-functionalized Pyrazoles.Chemistry. 2016 May 17;22(21):7059-62. doi: 10.1002/chem.201600704. Epub 2016 Apr 15. Chemistry. 2016. PMID: 26991450 Free PMC article.
-
Rational Development of Remote C-H Functionalization of Biphenyl: Experimental and Computational Studies.Angew Chem Int Ed Engl. 2020 Mar 16;59(12):4770-4777. doi: 10.1002/anie.201915624. Epub 2020 Feb 12. Angew Chem Int Ed Engl. 2020. PMID: 31943648 Free PMC article.
-
Directing Remote Meta-C-H Functionalization with Cleavable Auxiliaries.ACS Cent Sci. 2015 Nov 25;1(8):418-9. doi: 10.1021/acscentsci.5b00358. Epub 2015 Nov 11. ACS Cent Sci. 2015. PMID: 27163003 Free PMC article. No abstract available.
-
Rh(iii)-catalyzed C-H olefination of N-pentafluoroaryl benzamides using air as the sole oxidant.Chem Sci. 2015 Mar 1;6(3):1923-1927. doi: 10.1039/c4sc03350g. Epub 2015 Jan 8. Chem Sci. 2015. PMID: 29449919 Free PMC article.
-
Recent development in transition metal-catalysed C-H olefination.Chem Sci. 2021 Jan 29;12(8):2735-2759. doi: 10.1039/d0sc05555g. Chem Sci. 2021. PMID: 34164039 Free PMC article. Review.
References
-
-
For early discussion and other approaches for remote C–H activation: Breslow R. Acc Chem Res. 1980;13:170.Schwarz H. Acc Chem Res. 1989;22:282.Das S, Incarvito CD, Crabtree RH, Brudvig GW. Science. 2006;312:1941.
-
-
- Hennings DD, Iwasa S, Rawal VH. J Org Chem. 1997;62:2. - PubMed
- Satoh T, Kawamura Y, Miura M, Nomura M. Angew Chem, Int Ed. 1997;36:1740.
- Miura M, Tsuda T, Satoh T, Nomura M. Chem Lett. 1997:1103.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

