Pd(II)-catalyzed ortho- or meta-C-H olefination of phenol derivatives
- PMID: 23614807
- PMCID: PMC3685289
- DOI: 10.1021/ja400659s
Pd(II)-catalyzed ortho- or meta-C-H olefination of phenol derivatives
Abstract
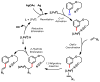
A combination of weakly coordinating auxiliaries and ligand acceleration allows for the development of both ortho- and meta-selective C-H olefination of phenol derivatives. These reactions demonstrate the feasibility of directing C-H functionalizations when functional groups are distal to target C-H bonds. The meta-C-H functionalization of electron-rich phenol derivatives is unprecedented and orthogonal to previous electrophilic substitution of phenols in terms of regioselectivity. These methods are also applied to functionalize α-phenoxyacetic acids, a fibrate class of drug scaffolds.
Figures

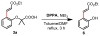
References
-
-
For early discussion and other approaches for remote C–H activation: Breslow R. Acc Chem Res. 1980;13:170.Schwarz H. Acc Chem Res. 1989;22:282.Das S, Incarvito CD, Crabtree RH, Brudvig GW. Science. 2006;312:1941.
-
-
- Hennings DD, Iwasa S, Rawal VH. J Org Chem. 1997;62:2. - PubMed
- Satoh T, Kawamura Y, Miura M, Nomura M. Angew Chem, Int Ed. 1997;36:1740.
- Miura M, Tsuda T, Satoh T, Nomura M. Chem Lett. 1997:1103.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

