Oxidative damage of DNA induced by the reaction of methylglyoxal with lysine in the presence of ferritin
- PMID: 23615265
- PMCID: PMC4133886
- DOI: 10.5483/bmbrep.2013.46.4.225
Oxidative damage of DNA induced by the reaction of methylglyoxal with lysine in the presence of ferritin
Abstract
Methylglyoxal (MG) is an endogenous metabolite which is present in increased concentrations in diabetics and reacts with amino acids to form advanced glycation end products. In this study, we investigated whether ferritin enhances DNA cleavage by the reaction of MG with lysine. When plasmid DNA was incubated with MG and lysine in the presence of ferritin, DNA strand breakage was increased in a dose-dependent manner. The ferritin/MG/lysine system-mediated DNA cleavage was significantly inhibited by reactive oxygen species (ROS) scavengers. These results indicated that ROS might participate in the ferritin/MG/lysine system-mediated DNA cleavage. Incubation of ferritin with MG and lysine resulted in a time-dependent release of iron ions from the protein molecules. Our data suggest that DNA cleavage caused by the ferritin/MG/lysine system via the generation of ROS by the Fenton-like reaction of free iron ions released from oxidatively damaged ferritin.
Figures
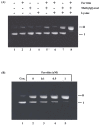
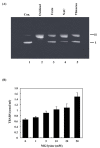
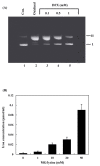
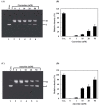
Similar articles
-
Oxidative damage of DNA by the reaction of amino acid with methylglyoxal in the presence of Fe(III).Int J Biol Macromol. 2003 Nov;33(1-3):43-8. doi: 10.1016/s0141-8130(03)00064-3. Int J Biol Macromol. 2003. PMID: 14599583
-
Oxidative modification of ferritin induced by methylglyoxal.BMB Rep. 2012 Mar;45(3):147-52. doi: 10.5483/BMBRep.2012.45.3.147. BMB Rep. 2012. PMID: 22449700
-
Oxidative damage of DNA induced by methylglyoxal in vitro.Toxicol Lett. 2003 Nov 30;145(2):181-7. doi: 10.1016/s0378-4274(03)00305-9. Toxicol Lett. 2003. PMID: 14581171
-
Nuclear ferritin in corneal epithelial cells: tissue-specific nuclear transport and protection from UV-damage.Prog Retin Eye Res. 2005 Mar;24(2):139-59. doi: 10.1016/j.preteyeres.2004.08.004. Epub 2004 Nov 11. Prog Retin Eye Res. 2005. PMID: 15610971 Review.
-
Molecular, physiological and clinical aspects of the iron storage protein ferritin.Vet J. 2008 Nov;178(2):191-201. doi: 10.1016/j.tvjl.2007.07.006. Epub 2007 Aug 30. Vet J. 2008. PMID: 17764995 Review.
Cited by
-
Cyanidin-3-rutinoside attenuates methylglyoxal-induced protein glycation and DNA damage via carbonyl trapping ability and scavenging reactive oxygen species.BMC Complement Altern Med. 2016 May 23;16:138. doi: 10.1186/s12906-016-1133-x. BMC Complement Altern Med. 2016. PMID: 27215203 Free PMC article.
-
Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging.Cells. 2019 Jul 19;8(7):749. doi: 10.3390/cells8070749. Cells. 2019. PMID: 31331077 Free PMC article. Review.
-
Enhancement of UVB radiation-mediated apoptosis by knockdown of cytosolic NADP+-dependent isocitrate dehydrogenase in HaCaT cells.BMB Rep. 2014 Apr;47(4):209-14. doi: 10.5483/bmbrep.2014.47.4.137. BMB Rep. 2014. PMID: 24286310 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

