Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions
- PMID: 23615497
- PMCID: PMC3778762
- DOI: 10.1038/ki.2013.114
Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions
Abstract
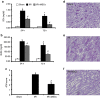
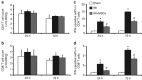
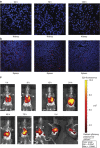
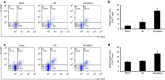
The mechanism of mesenchymal stem cell therapy in acute kidney injury remains uncertain. Previous studies indicated that mesenchymal stem cells could attenuate inflammation-related organ injury by induction of regulatory T cells. Whether regulatory T-cell induction is a potential mechanism of mesenchymal stem cell therapy in ischemic acute kidney injury and how these induced regulatory T cells orchestrate local inflammation are unknown. Here we found that mesenchymal stem cells decrease serum creatinine and urea nitrogen levels, improve tubular injury, and downregulate IFN-γ production of T cells in the ischemic kidney. In addition to the lung, mesenchymal stem cells persisted mostly in the spleen. Mesenchymal stem cells increased the percentage of regulatory T cells in the spleen and the ischemic kidney. Antibody-dependent depletion of regulatory T cells blunted the therapeutic effect of mesenchymal stem cells, while coculture of splenocytes with mesenchymal stem cells caused an increase in the percentage of regulatory T cells. Splenectomy abrogated attenuation of ischemic injury, and downregulated IFN-γ production and the induction of regulatory T cells by mesenchymal stem cells. Thus, mesenchymal stem cells ameliorate ischemic acute kidney injury by inducing regulatory T cells through interactions with splenocytes. Accumulated regulatory T cells in ischemic kidney might be involved in the downregulation of IFN-γ production.
Figures


Comment in
-
Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions.Kidney Int. 2014 Apr;85(4):981-2. doi: 10.1038/ki.2013.562. Kidney Int. 2014. PMID: 24682126 No abstract available.
Similar articles
-
Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions.Kidney Int. 2014 Apr;85(4):981-2. doi: 10.1038/ki.2013.562. Kidney Int. 2014. PMID: 24682126 No abstract available.
-
IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway.Kidney Int. 2018 Apr;93(4):814-825. doi: 10.1016/j.kint.2017.08.030. Epub 2017 Nov 11. Kidney Int. 2018. PMID: 29132705
-
Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury.Exp Biol Med (Maywood). 2013 Aug 1;238(8):960-70. doi: 10.1177/1535370213497176. Exp Biol Med (Maywood). 2013. PMID: 23970411
-
Mesenchymal Stem Cells in Renal Ischemia-Reperfusion Injury: Biological and Therapeutic Perspectives.Curr Stem Cell Res Ther. 2017;12(3):183-187. doi: 10.2174/1574888X11666161024143640. Curr Stem Cell Res Ther. 2017. PMID: 27781940 Review.
-
Novel preconditioning strategies for enhancing the migratory ability of mesenchymal stem cells in acute kidney injury.Stem Cell Res Ther. 2018 Aug 23;9(1):225. doi: 10.1186/s13287-018-0973-3. Stem Cell Res Ther. 2018. PMID: 30139368 Free PMC article. Review.
Cited by
-
Cell-based therapy for kidney disease.Korean J Urol. 2015 Jun;56(6):412-21. doi: 10.4111/kju.2015.56.6.412. Epub 2015 May 27. Korean J Urol. 2015. PMID: 26078837 Free PMC article. Review.
-
Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway.Front Physiol. 2020 Apr 3;11:285. doi: 10.3389/fphys.2020.00285. eCollection 2020. Front Physiol. 2020. PMID: 32317985 Free PMC article.
-
Stem cell delivery to kidney via minimally invasive ultrasound-guided renal artery injection in mice.Sci Rep. 2020 May 5;10(1):7514. doi: 10.1038/s41598-020-64417-2. Sci Rep. 2020. PMID: 32372054 Free PMC article.
-
Exogenous pericyte delivery protects the mouse kidney from chronic ischemic injury.Am J Physiol Renal Physiol. 2022 Nov 1;323(5):F527-F538. doi: 10.1152/ajprenal.00064.2022. Epub 2022 Sep 1. Am J Physiol Renal Physiol. 2022. PMID: 36049063 Free PMC article.
-
Preclinical assessments of safety and tumorigenicity of very high doses of allogeneic human umbilical cord mesenchymal stem cells.In Vitro Cell Dev Biol Anim. 2024 Mar;60(3):307-319. doi: 10.1007/s11626-024-00852-z. Epub 2024 Feb 29. In Vitro Cell Dev Biol Anim. 2024. PMID: 38421574 Free PMC article.
References
-
- Palevsky PM. Epidemiology of acute renal failure: the tip of the iceberg. Clin J Am Soc Nephrol. 2006;1:6–7. - PubMed
-
- Semedo P, Palasio CG, Oliveira CD, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–682. - PubMed
-
- Zhuo W, Liao L, Xu T, et al. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86:191–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

