Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy
- PMID: 23615526
- PMCID: PMC3720846
- DOI: 10.1158/1541-7786.MCR-12-0600
Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy
Abstract
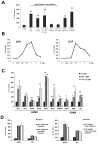
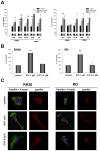
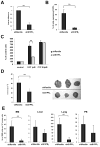
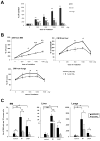
Evidence suggests that bioactive lipids may regulate pathophysiologic functions such as cancer cell metastasis. Therefore, we determined that the bioactive lipid chemoattractants sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) strongly enhanced the in vitro motility and adhesion of human rhabdomyosarcoma (RMS) cells. Importantly, this effect was observed at physiologic concentrations for both bioactive lipids, which are present in biologic fluids, and were much stronger than the effects observed in response to known RMS prometastatic factors such as stromal derived factors-1 (SDF-1/CXCL12) or hepatocyte growth factor/scatter factor (HGF/SF). We also present novel evidence that the levels of S1P and C1P were increased in several organs after γ-irradiation or chemotherapy, which indicates an unwanted prometastatic environment related to treatment. Critically, we found that the metastasis of RMS cells in response to S1P can be effectively inhibited in vivo with the S1P-specific binder NOX-S93 that is based on a high-affinity Spiegelmer. These data indicate that bioactive lipids play a vital role in dissemination of RMS and contribute to the unwanted side effects of radio/chemotherapy by creating a prometastatic microenvironment.
©2013 AACR
Conflict of interest statement
Conflict of Interest: Gabriela Schneider and Mariusz Ratajczak received minor commercial research funding from NOXXON Pharma AG for studying the effect of NOX-S93 in chemo-radiation induced metastasis of RMS.
Figures


Similar articles
-
Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy.Mol Cancer Res. 2014 Nov;12(11):1560-73. doi: 10.1158/1541-7786.MCR-14-0188. Epub 2014 Jul 17. Mol Cancer Res. 2014. PMID: 25033840 Free PMC article.
-
Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy.Cancer Res. 2003 Nov 15;63(22):7926-35. Cancer Res. 2003. PMID: 14633723
-
Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow-derived stem cells in patients with acute myocardial infarction.Stem Cells Dev. 2013 Jun 1;22(11):1645-56. doi: 10.1089/scd.2012.0488. Epub 2013 Feb 19. Stem Cells Dev. 2013. PMID: 23282236 Free PMC article.
-
Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling.J Cell Sci. 2005 Oct 15;118(Pt 20):4605-12. doi: 10.1242/jcs.02637. J Cell Sci. 2005. PMID: 16219683 Review.
-
The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer.Mediators Inflamm. 2017;2017:4806541. doi: 10.1155/2017/4806541. Epub 2017 Nov 15. Mediators Inflamm. 2017. PMID: 29269995 Free PMC article. Review.
Cited by
-
Distinctive sphingolipid patterns in chronic multiple sclerosis lesions.J Lipid Res. 2020 Nov;61(11):1464-1479. doi: 10.1194/jlr.RA120001022. Epub 2020 Aug 7. J Lipid Res. 2020. PMID: 32769146 Free PMC article.
-
Evidence that vitronectin is a potent migration-enhancing factor for cancer cells chaperoned by fibrinogen: a novel view of the metastasis of cancer cells to low-fibrinogen lymphatics and body cavities.Oncotarget. 2016 Oct 25;7(43):69829-69843. doi: 10.18632/oncotarget.12003. Oncotarget. 2016. PMID: 27634880 Free PMC article.
-
Sphingosine-1-Phosphate Recruits Macrophages and Microglia and Induces a Pro-Tumorigenic Phenotype That Favors Glioma Progression.Cancers (Basel). 2023 Jan 12;15(2):479. doi: 10.3390/cancers15020479. Cancers (Basel). 2023. PMID: 36672428 Free PMC article.
-
Mobilization studies in mice deficient in sphingosine kinase 2 support a crucial role of the plasma level of sphingosine-1-phosphate in the egress of hematopoietic stem progenitor cells.Oncotarget. 2017 Jul 24;8(39):65588-65600. doi: 10.18632/oncotarget.19514. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029455 Free PMC article.
-
Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy.Mol Cancer Res. 2014 Nov;12(11):1560-73. doi: 10.1158/1541-7786.MCR-14-0188. Epub 2014 Jul 17. Mol Cancer Res. 2014. PMID: 25033840 Free PMC article.
References
-
- Collins MH, Zhao H, Womer RB, Barr FG. Proliferative and apoptotic differences between alveolar rhabdomyosarcoma subtypes: a comparative study of tumors containing PAX3-FKHR or PAX7-FKHR gene fusions. Med Pediatr Oncol. 2001;37(2):83–9. - PubMed
-
- Sandberg AA, Stone JF, Czarnecki L, Cohen JD. Hematologic masquerade of rhabdomyosarcoma. Am J Hematol. 2001;68(1):51–7. - PubMed
-
- Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994;54(11):2869–72. - PubMed
-
- Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100(7):2597–606. - PubMed
-
- Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, et al. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63(22):7926–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

