GABAergic transmission facilitates ictogenesis and synchrony between CA3, hilus, and dentate gyrus in slices from epileptic rats
- PMID: 23615549
- PMCID: PMC3727068
- DOI: 10.1152/jn.00679.2012
GABAergic transmission facilitates ictogenesis and synchrony between CA3, hilus, and dentate gyrus in slices from epileptic rats
Abstract
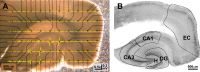
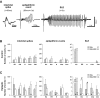
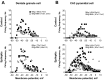
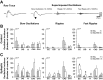
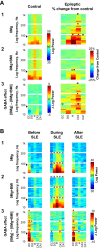
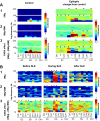
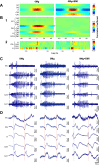
The impact of regional hippocampal interactions and GABAergic transmission on ictogenesis remain unclear. Cortico-hippocampal slices from pilocarpine-treated epileptic rats were compared with controls to investigate associations between seizurelike events (SLE), GABAergic transmission, and neuronal synchrony within and between cortico-hippocampal regions. Multielectrode array recordings revealed more prevalent hippocampal SLE in epileptic tissue when excitatory transmission was enhanced and GABAergic transmission was intact [removal of Mg(2+) (0Mg)] than when GABAergic transmission was blocked [removal of Mg(2+) + bicuculline methiodide (0Mg+BMI)]. When activity within individual regions was analyzed, spectral and temporal slow oscillation/SLE correlations and cross-correlations were highest within the hilus of epileptic tissue during SLE but were similar in 0Mg and 0Mg+BMI. GABAergic facilitation of spectral "slow" oscillation and ripple correlations was most prominent within CA3 of epileptic tissue during SLE. When activity between regions was analyzed, slow oscillation and ripple coherence was highest between the hilus and dentate gyrus as well as between the hilus and CA3 of epileptic tissue during SLE and was significantly higher in 0Mg than 0Mg+BMI. High 0Mg-induced SLE cross-correlations between the hilus and dentate gyrus as well as between the hilus and CA3 were reduced or abolished in 0Mg+BMI. SLE cross-correlation lag measurements provided evidence for a monosynaptic connection from the hilus to the dentate gyrus during SLE. Findings implicate the hilus as an oscillation generator, whose impact on other cortico-hippocampal regions is mediated by GABAergic transmission. Data also suggest that GABAA receptor-mediated transmission facilitates back-propagation from CA3/hilus to the dentate gyrus and that this back-propagation augments SLE in epileptic hippocampus.
Keywords: CA1; electrophysiology; entorhinal cortex; epilepsy; hilus; hippocampus; multielectrode array.
Figures
Similar articles
-
Causal relationship of CA3 back-projection to the dentate gyrus and its role in CA1 fast ripple generation.BMC Neurosci. 2021 May 17;22(1):37. doi: 10.1186/s12868-021-00641-4. BMC Neurosci. 2021. PMID: 34001031 Free PMC article.
-
Decrement of GABAA receptor-mediated inhibitory postsynaptic currents in dentate granule cells in epileptic hippocampus.J Neurophysiol. 1996 May;75(5):1901-8. doi: 10.1152/jn.1996.75.5.1901. J Neurophysiol. 1996. PMID: 8734589
-
Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats.J Neurophysiol. 1998 Jan;79(1):418-29. doi: 10.1152/jn.1998.79.1.418. J Neurophysiol. 1998. PMID: 9425210
-
Epileptogenesis in the dentate gyrus: a critical perspective.Prog Brain Res. 2007;163:755-73. doi: 10.1016/S0079-6123(07)63041-6. Prog Brain Res. 2007. PMID: 17765749 Review.
-
Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro.Prog Neurobiol. 2002 Oct;68(3):167-207. doi: 10.1016/s0301-0082(02)00077-1. Prog Neurobiol. 2002. PMID: 12450487 Review.
Cited by
-
Early Appearance and Spread of Fast Ripples in the Hippocampus in a Model of Cortical Traumatic Brain Injury.J Neurosci. 2018 Oct 17;38(42):9034-9046. doi: 10.1523/JNEUROSCI.3507-17.2018. Epub 2018 Sep 6. J Neurosci. 2018. PMID: 30190413 Free PMC article.
-
Synaptic plasticity in glutamatergic and GABAergic neurotransmission following chronic memantine treatment in an in vitro model of limbic epileptogenesis.Neuropharmacology. 2014 Feb;77:379-86. doi: 10.1016/j.neuropharm.2013.10.016. Epub 2013 Oct 30. Neuropharmacology. 2014. PMID: 24184417 Free PMC article.
-
Examining the low-voltage fast seizure-onset and its response to optogenetic stimulation in a biophysical network model of the hippocampus.Cogn Neurodyn. 2024 Feb;18(1):265-282. doi: 10.1007/s11571-023-09935-1. Epub 2023 Feb 10. Cogn Neurodyn. 2024. PMID: 38406204 Free PMC article.
-
Rescue of sharp wave-ripples and prevention of network hyperexcitability in the ventral but not the dorsal hippocampus of a rat model of fragile X syndrome.Front Cell Neurosci. 2023 Dec 1;17:1296235. doi: 10.3389/fncel.2023.1296235. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38107412 Free PMC article.
-
Chronic reduction of synaptic proteins in the epileptogenic lesion of patients with hippocampal sclerosis.Front Mol Neurosci. 2025 Jul 23;18:1635852. doi: 10.3389/fnmol.2025.1635852. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40771360 Free PMC article.
References
-
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: The Hippocampus Book, edited by Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. Oxford, UK: Oxford Univ. Press, 2007
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591, 1989 - PubMed
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182: 851–914, 1978 - PubMed
-
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res 398: 215–219, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

