The A128T resistance mutation reveals aberrant protein multimerization as the primary mechanism of action of allosteric HIV-1 integrase inhibitors
- PMID: 23615903
- PMCID: PMC3668738
- DOI: 10.1074/jbc.M112.443390
The A128T resistance mutation reveals aberrant protein multimerization as the primary mechanism of action of allosteric HIV-1 integrase inhibitors
Abstract
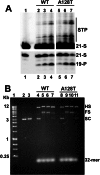
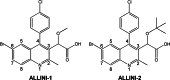
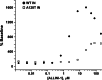
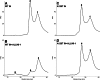
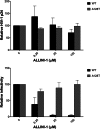
Allosteric HIV-1 integrase (IN) inhibitors (ALLINIs) are a very promising new class of anti-HIV-1 agents that exhibit a multimodal mechanism of action by allosterically modulating IN multimerization and interfering with IN-lens epithelium-derived growth factor (LEDGF)/p75 binding. Selection of viral strains under ALLINI pressure has revealed an A128T substitution in HIV-1 IN as a primary mechanism of resistance. Here, we elucidated the structural and mechanistic basis for this resistance. The A128T substitution did not affect the hydrogen bonding between ALLINI and IN that mimics the IN-LEDGF/p75 interaction but instead altered the positioning of the inhibitor at the IN dimer interface. Consequently, the A128T substitution had only a minor effect on the ALLINI IC50 values for IN-LEDGF/p75 binding. Instead, ALLINIs markedly altered the multimerization of IN by promoting aberrant higher order WT (but not A128T) IN oligomers. Accordingly, WT IN catalytic activities and HIV-1 replication were potently inhibited by ALLINIs, whereas the A128T substitution in IN resulted in significant resistance to the inhibitors both in vitro and in cell culture assays. The differential multimerization of WT and A128T INs induced by ALLINIs correlated with the differences in infectivity of HIV-1 progeny virions. We conclude that ALLINIs primarily target IN multimerization rather than IN-LEDGF/p75 binding. Our findings provide the structural foundations for developing improved ALLINIs with increased potency and decreased potential to select for drug resistance.
Keywords: Allosteric Inhibitors; Drug Resistance; HIV-1; Infectious Diseases; Integrase; Pharmacology; Protein-DNA Interactions; Protein-Drug Integrations; Protein-Protein Interactions; Structural Biology.
Figures


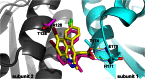
Similar articles
-
Multimodal mechanism of action of allosteric HIV-1 integrase inhibitors.Expert Rev Mol Med. 2013 Nov 26;15:e14. doi: 10.1017/erm.2013.15. Expert Rev Mol Med. 2013. PMID: 24274067 Free PMC article. Review.
-
The structural and mechanistic bases for the viral resistance to allosteric HIV-1 integrase inhibitor pirmitegravir.mBio. 2024 Nov 13;15(11):e0046524. doi: 10.1128/mbio.00465-24. Epub 2024 Oct 15. mBio. 2024. PMID: 39404354 Free PMC article.
-
The Competitive Interplay between Allosteric HIV-1 Integrase Inhibitor BI/D and LEDGF/p75 during the Early Stage of HIV-1 Replication Adversely Affects Inhibitor Potency.ACS Chem Biol. 2016 May 20;11(5):1313-21. doi: 10.1021/acschembio.6b00167. Epub 2016 Mar 2. ACS Chem Biol. 2016. PMID: 26910179 Free PMC article.
-
A critical role of the C-terminal segment for allosteric inhibitor-induced aberrant multimerization of HIV-1 integrase.J Biol Chem. 2014 Sep 19;289(38):26430-26440. doi: 10.1074/jbc.M114.589572. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118283 Free PMC article.
-
Allosteric inhibition of HIV-1 integrase activity.Curr Opin Chem Biol. 2013 Jun;17(3):339-45. doi: 10.1016/j.cbpa.2013.04.010. Epub 2013 May 3. Curr Opin Chem Biol. 2013. PMID: 23647983 Free PMC article. Review.
Cited by
-
Allosteric HIV-1 integrase inhibitors promote aberrant protein multimerization by directly mediating inter-subunit interactions: Structural and thermodynamic modeling studies.Protein Sci. 2016 Nov;25(11):1911-1917. doi: 10.1002/pro.2997. Epub 2016 Aug 17. Protein Sci. 2016. PMID: 27503276 Free PMC article.
-
Drug Resistance to Integrase Strand-Transfer Inhibitors among HIV-1-Infected Adults in Guangdong, China.Pathogens. 2022 Nov 10;11(11):1321. doi: 10.3390/pathogens11111321. Pathogens. 2022. PMID: 36365072 Free PMC article.
-
An Isoquinoline Scaffold as a Novel Class of Allosteric HIV-1 Integrase Inhibitors.ACS Med Chem Lett. 2019 Jan 30;10(2):215-220. doi: 10.1021/acsmedchemlett.8b00633. eCollection 2019 Feb 14. ACS Med Chem Lett. 2019. PMID: 30783506 Free PMC article.
-
Multimodal mechanism of action of allosteric HIV-1 integrase inhibitors.Expert Rev Mol Med. 2013 Nov 26;15:e14. doi: 10.1017/erm.2013.15. Expert Rev Mol Med. 2013. PMID: 24274067 Free PMC article. Review.
-
A new class of multimerization selective inhibitors of HIV-1 integrase.PLoS Pathog. 2014 May 29;10(5):e1004171. doi: 10.1371/journal.ppat.1004171. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24874515 Free PMC article.
References
-
- Johnson A. A., Marchand C., Pommier Y. (2004) HIV-1 integrase inhibitors: a decade of research and two drugs in clinical trial. Curr. Top. Med. Chem. 4, 1059–1077 - PubMed
-
- Brown P. O. (1997) Integration. in Retroviruses (Coffin J. M., Hughes S. H., Varmus H. E. eds) pp. 161–204, Cold Spring Harbor Laboratory, Plainview, NY
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

