Probing novel roles of the mitochondrial uniporter in ovarian cancer cells using nanoparticles
- PMID: 23615904
- PMCID: PMC3682561
- DOI: 10.1074/jbc.M112.435206
Probing novel roles of the mitochondrial uniporter in ovarian cancer cells using nanoparticles
Abstract
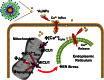
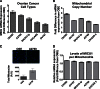
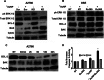
Nanoparticles provide a potent tool for targeting and understanding disease mechanisms. In this regard, cancer cells are surprisingly resistant to the expected toxic effects of positively charged gold nanoparticles ((+)AuNPs). Our investigations led to the identification of MICU1, regulator of mitochondrial calcium uniporter, as a key molecule conferring cancer cells with resistance to (+)AuNPs. The increase in cytosolic [Ca(2+)]cyto in malignant cells induced by (+)AuNPs is counteracted by MICU1, preventing cell death. Pharmacological or siRNA-mediated inhibition of mitochondrial Ca(+2) entry leads to endoplasmic reticulum stress and sensitizes cancer cells to (+)AuNP-induced cytotoxicity. Silencing MICU1 decreases Bcl-2 expression and increases caspase-3 activity and cytosolic cytochrome c levels, thus initiating the mitochondrial pathway for apoptosis: effects further enhanced by (+)AuNPs. This study highlights the potential of nanomaterials as a tool to broaden our understanding of cellular processes, establishes MICU1 as a novel regulator of the machinery in cancer cells that prevents apoptosis, and emphasizes the need to synergize nanoparticle design with understanding of mitochondrial machinery for enhancing targeted cellular toxicity.
Keywords: Apoptosis; Bcl-2; Cancer; Cancer Biology; Caspase; Mitochondrial Apoptosis.
Figures




Similar articles
-
Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca(2.).Sci Rep. 2015 Oct 22;5:15602. doi: 10.1038/srep15602. Sci Rep. 2015. PMID: 26489515 Free PMC article.
-
Functional roles of MICU1 and MICU2 in mitochondrial Ca(2+) uptake.Biochim Biophys Acta. 2016 Jun;1858(6):1110-7. doi: 10.1016/j.bbamem.2016.02.022. Epub 2016 Feb 18. Biochim Biophys Acta. 2016. PMID: 26903221
-
Mitochondrial calcium uniporter activity is dispensable for MDA-MB-231 breast carcinoma cell survival.PLoS One. 2014 May 6;9(5):e96866. doi: 10.1371/journal.pone.0096866. eCollection 2014. PLoS One. 2014. PMID: 24802861 Free PMC article.
-
MICU1's calcium sensing beyond mitochondrial calcium uptake.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119714. doi: 10.1016/j.bbamcr.2024.119714. Epub 2024 Mar 29. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38555977 Free PMC article. Review.
-
The In Vivo Biology of the Mitochondrial Calcium Uniporter.Adv Exp Med Biol. 2017;982:49-63. doi: 10.1007/978-3-319-55330-6_3. Adv Exp Med Biol. 2017. PMID: 28551781 Review.
Cited by
-
Interactions of nanomaterials with ion channels and related mechanisms.Br J Pharmacol. 2019 Oct;176(19):3754-3774. doi: 10.1111/bph.14792. Epub 2019 Sep 4. Br J Pharmacol. 2019. PMID: 31290152 Free PMC article. Review.
-
Multiparametric analysis of anti-proliferative and apoptotic effects of gold nanoprisms on mouse and human primary and transformed cells, biodistribution and toxicity in vivo.Part Fibre Toxicol. 2017 Oct 26;14(1):41. doi: 10.1186/s12989-017-0222-4. Part Fibre Toxicol. 2017. PMID: 29073907 Free PMC article.
-
Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment.Br J Pharmacol. 2019 May;176(9):1190-1205. doi: 10.1111/bph.14632. Epub 2019 Apr 3. Br J Pharmacol. 2019. PMID: 30801705 Free PMC article. Review.
-
MICU1 drives glycolysis and chemoresistance in ovarian cancer.Nat Commun. 2017 May 22;8:14634. doi: 10.1038/ncomms14634. Nat Commun. 2017. PMID: 28530221 Free PMC article.
-
Mitochondrial dysfunction induces radioresistance in colorectal cancer by activating [Ca2+]m-PDP1-PDH-histone acetylation retrograde signaling.Cell Death Dis. 2021 Sep 6;12(9):837. doi: 10.1038/s41419-021-03984-2. Cell Death Dis. 2021. PMID: 34489398 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

