Influence of trial sample size on treatment effect estimates: meta-epidemiological study
- PMID: 23616031
- PMCID: PMC3634626
- DOI: 10.1136/bmj.f2304
Influence of trial sample size on treatment effect estimates: meta-epidemiological study
Abstract
Objective: To assess the influence of trial sample size on treatment effect estimates within meta-analyses.
Design: Meta-epidemiological study.
Data sources: 93 meta-analyses (735 randomised controlled trials) assessing therapeutic interventions with binary outcomes, published in the 10 leading journals of each medical subject category of the Journal Citation Reports or in the Cochrane Database of Systematic Reviews.
Data extraction: Sample size, outcome data, and risk of bias extracted from each trial.
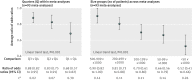
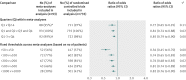
Data synthesis: Trials within each meta-analysis were sorted by their sample size: using quarters within each meta-analysis (from quarter 1 with 25% of the smallest trials, to quarter 4 with 25% of the largest trials), and using size groups across meta-analyses (ranging from <50 to ≥ 1000 patients). Treatment effects were compared within each meta-analysis between quarters or between size groups by average ratios of odds ratios (where a ratio of odds ratios less than 1 indicates larger effects in smaller trials).
Results: Treatment effect estimates were significantly larger in smaller trials, regardless of sample size. Compared with quarter 4 (which included the largest trials), treatment effects were, on average, 32% larger in trials in quarter 1 (which included the smallest trials; ratio of odds ratios 0.68, 95% confidence interval 0.57 to 0.82), 17% larger in trials in quarter 2 (0.83, 0.75 to 0.91), and 12% larger in trials in quarter 3 (0.88, 0.82 to 0.95). Similar results were obtained when comparing treatment effect estimates between different size groups. Compared with trials of 1000 patients or more, treatment effects were, on average, 48% larger in trials with fewer than 50 patients (0.52, 0.41 to 0.66) and 10% larger in trials with 500-999 patients (0.90, 0.82 to 1.00).
Conclusions: Treatment effect estimates differed within meta-analyses solely based on trial sample size, with stronger effect estimates seen in small to moderately sized trials than in the largest trials.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Brandler E, Paladino L, Sinert R. Does the early administration of beta-blockers improve the in-hospital mortality rate of patients admitted with acute coronary syndrome? Acad Emerg Med 2010;17:1-10. - PubMed
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119-29. - PubMed
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
