Coordinated interactions of multiple POT1-TPP1 proteins with telomere DNA
- PMID: 23616058
- PMCID: PMC3675573
- DOI: 10.1074/jbc.M113.471896
Coordinated interactions of multiple POT1-TPP1 proteins with telomere DNA
Abstract
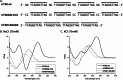
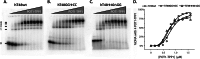
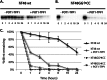
Telomeres are macromolecular nucleoprotein complexes that protect the ends of eukaryotic chromosomes from degradation, end-to-end fusion events, and from engaging the DNA damage response. However, the assembly of this essential DNA-protein complex is poorly understood. Telomere DNA consists of the repeated double-stranded sequence 5'-TTAGGG-3' in vertebrates, followed by a single-stranded DNA overhang with the same sequence. Both double- and single-stranded regions are coated with high specificity by telomere end-binding proteins, including POT1 and TPP1, that bind as a heterodimer to single-stranded telomeric DNA. Multiple POT1-TPP1 proteins must fully coat the single-stranded telomere DNA to form a functional telomere. To better understand the mechanism of multiple binding, we mutated or deleted the two guanosine nucleotides residing between adjacent POT1-TPP1 recognition sites in single-stranded telomere DNA that are not required for multiple POT1-TPP1 binding events. Circular dichroism demonstrated that spectra from the native telomere sequence are characteristic of a G-quadruplex secondary structure, whereas the altered telomere sequences were devoid of these signatures. The altered telomere strands, however, facilitated more cooperative loading of multiple POT1-TPP1 proteins compared with the wild-type telomere sequence. Finally, we show that a 48-nucleotide DNA with a telomere sequence is more susceptible to nuclease digestion when coated with POT1-TPP1 proteins than when it is left uncoated. Together, these data suggest that POT1-TPP1 binds telomeric DNA in a coordinated manner to facilitate assembly of the nucleoprotein complexes into a state that is more accessible to enzymatic activity.
Keywords: DNA Binding Protein; DNA Structure; G-quadruplex; Nucleotide; POT1; TPP1; Telomerase; Telomeres.
Figures

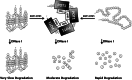
Similar articles
-
TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase.Nature. 2007 Feb 1;445(7127):559-62. doi: 10.1038/nature05469. Epub 2007 Jan 21. Nature. 2007. PMID: 17237767
-
POT1-TPP1 Binding and Unfolding of Telomere DNA Discriminates against Structural Polymorphism.J Mol Biol. 2016 Jul 3;428(13):2695-708. doi: 10.1016/j.jmb.2016.04.031. Epub 2016 May 10. J Mol Biol. 2016. PMID: 27173378 Free PMC article.
-
Multiple POT1-TPP1 proteins coat and compact long telomeric single-stranded DNA.J Mol Biol. 2011 Jul 1;410(1):10-7. doi: 10.1016/j.jmb.2011.04.049. Epub 2011 May 9. J Mol Biol. 2011. PMID: 21596049 Free PMC article.
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
Comparison of Telomere Structure in Eukaryotes.Arch Razi Inst. 2024 Dec 31;79(6):1365-1374. doi: 10.32592/ARI.2024.79.6.1365. eCollection 2024 Dec. Arch Razi Inst. 2024. PMID: 40606259 Free PMC article. Review.
Cited by
-
Shelterin complex gene: Prognosis and therapeutic vulnerability in cancer.Biochem Biophys Rep. 2021 Feb 2;26:100937. doi: 10.1016/j.bbrep.2021.100937. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33553693 Free PMC article. Review.
-
Telomere DNA G-quadruplex folding within actively extending human telomerase.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9350-9359. doi: 10.1073/pnas.1814777116. Epub 2019 Apr 24. Proc Natl Acad Sci U S A. 2019. PMID: 31019071 Free PMC article.
-
Multiple hPOT1-TPP1 cooperatively unfold contiguous telomeric G-quadruplexes proceeding from 3' toward 5', a feature due to a 3'-end binding preference and to structuring of telomeric DNA.Nucleic Acids Res. 2021 Oct 11;49(18):10735-10746. doi: 10.1093/nar/gkab768. Nucleic Acids Res. 2021. PMID: 34534331 Free PMC article.
-
Protection of the Telomeric Junction by the Shelterin Complex.bioRxiv [Preprint]. 2024 Aug 19:2024.08.18.608453. doi: 10.1101/2024.08.18.608453. bioRxiv. 2024. Update in: J Am Chem Soc. 2024 Sep 11;146(36):25158-25165. doi: 10.1021/jacs.4c08649. PMID: 39229120 Free PMC article. Updated. Preprint.
-
Protection of the Telomeric Junction by the Shelterin Complex.J Am Chem Soc. 2024 Sep 11;146(36):25158-25165. doi: 10.1021/jacs.4c08649. Epub 2024 Aug 29. J Am Chem Soc. 2024. PMID: 39207958
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

