Caught in the act: revealing the metastatic process by live imaging
- PMID: 23616077
- PMCID: PMC3634643
- DOI: 10.1242/dmm.009282
Caught in the act: revealing the metastatic process by live imaging
Abstract
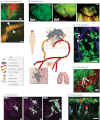
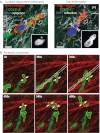
The prognosis of metastatic cancer in patients is poor. Interfering with metastatic spread is therefore important for achieving better survival from cancer. Metastatic disease is established through a series of steps, including breaching of the basement membrane, intravasation and survival in lymphatic or blood vessels, extravasation, and growth at distant sites. Yet, although we know the steps involved in metastasis, the cellular and molecular mechanisms of dissemination and colonization of distant organs are incompletely understood. Here, we review the important insights into the metastatic process that have been gained specifically through the use of imaging technologies in murine, chicken embryo and zebrafish model systems, including high-resolution two-photon microscopy and bioluminescence. We further discuss how imaging technologies are beginning to allow researchers to address the role of regional activation of specific molecular pathways in the metastatic process. These technologies are shedding light, literally, on almost every step of the metastatic process, particularly with regards to the dynamics and plasticity of the disseminating cancer cells and the active participation of the microenvironment in the processes.
Figures


References
-
- American Cancer Society (2011). Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society
-
- Bakker G. J., Andresen V., Hoffman R. M., Friedl P. (2012). Fluorescence lifetime microscopy of tumor cell invasion, drug delivery, and cytotoxicity. Methods Enzymol. 504, 109–125 - PubMed
-
- Bekes E. M., Schweighofer B., Kupriyanova T. A., Zajac E., Ardi V. C., Quigley J. P., Deryugina E. I. (2011). Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 179, 1455–1470 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

