Multiple sources of striatal inhibition are differentially affected in Huntington's disease mouse models
- PMID: 23616545
- PMCID: PMC3686572
- DOI: 10.1523/JNEUROSCI.2137-12.2013
Multiple sources of striatal inhibition are differentially affected in Huntington's disease mouse models
Abstract
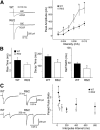
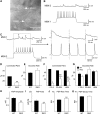
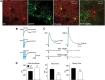
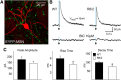
In Huntington's disease (HD) mouse models, spontaneous inhibitory synaptic activity is enhanced in a subpopulation of medium-sized spiny neurons (MSNs), which could dampen striatal output. We examined the potential source(s) of increased inhibition using electrophysiological and optogenetic methods to assess feedback and feedforward inhibition in two transgenic mouse models of HD. Single whole-cell patch-clamp recordings demonstrated that increased GABA synaptic activity impinges principally on indirect pathway MSNs. Dual patch recordings between MSNs demonstrated reduced connectivity between MSNs in HD mice. However, while connectivity was strictly unidirectional in controls, in HD mice bidirectional connectivity occurred. Other sources of increased GABA activity in MSNs also were identified. Dual patch recordings from fast spiking (FS) interneuron-MSN pairs demonstrated greater but variable amplitude responses in MSNs. In agreement, selective optogenetic stimulation of parvalbumin-expressing, FS interneurons induced significantly larger amplitude MSN responses in HD compared with control mice. While there were no differences in responses of MSNs evoked by activating single persistent low-threshold spiking (PLTS) interneurons in recorded pairs, these interneurons fired more action potentials in both HD models, providing another source for increased frequency of spontaneous GABA synaptic activity in MSNs. Selective optogenetic stimulation of somatostatin-expressing, PLTS interneurons did not reveal any significant differences in responses of MSNs in HD mice. These findings provide strong evidence that both feedforward and to a lesser extent feedback inhibition to MSNs in HD can potentially be sources for the increased GABA synaptic activity of indirect pathway MSNs.
Figures







Similar articles
-
Striatal Direct and Indirect Pathway Output Structures Are Differentially Altered in Mouse Models of Huntington's Disease.J Neurosci. 2018 May 16;38(20):4678-4694. doi: 10.1523/JNEUROSCI.0434-18.2018. Epub 2018 Apr 24. J Neurosci. 2018. PMID: 29691329 Free PMC article.
-
Target selectivity of feedforward inhibition by striatal fast-spiking interneurons.J Neurosci. 2013 Jan 23;33(4):1678-83. doi: 10.1523/JNEUROSCI.3572-12.2013. J Neurosci. 2013. PMID: 23345240 Free PMC article.
-
Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways.J Neurosci. 2010 Mar 3;30(9):3499-507. doi: 10.1523/JNEUROSCI.5139-09.2010. J Neurosci. 2010. PMID: 20203210 Free PMC article.
-
Functional Differences Between Direct and Indirect Striatal Output Pathways in Huntington's Disease.J Huntingtons Dis. 2012;1(1):17-25. doi: 10.3233/JHD-2012-120009. J Huntingtons Dis. 2012. PMID: 25063187 Free PMC article. Review.
-
Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function.Neuroscience. 2011 Dec 15;198:252-73. doi: 10.1016/j.neuroscience.2011.08.052. Epub 2011 Aug 27. Neuroscience. 2011. PMID: 21907762 Free PMC article. Review.
Cited by
-
Functional Autapses Form in Striatal Parvalbumin Interneurons but not Medium Spiny Projection Neurons.Neurosci Bull. 2023 Apr;39(4):576-588. doi: 10.1007/s12264-022-00991-x. Epub 2022 Dec 11. Neurosci Bull. 2023. PMID: 36502511 Free PMC article.
-
Synaptic Dysfunction in Huntington's Disease: Lessons from Genetic Animal Models.Neuroscientist. 2022 Feb;28(1):20-40. doi: 10.1177/1073858420972662. Epub 2020 Nov 16. Neuroscientist. 2022. PMID: 33198566 Free PMC article.
-
Optogenetics: 10 years of microbial opsins in neuroscience.Nat Neurosci. 2015 Sep;18(9):1213-25. doi: 10.1038/nn.4091. Nat Neurosci. 2015. PMID: 26308982 Free PMC article. Review.
-
Altered membrane properties and firing patterns of external globus pallidus neurons in the R6/2 mouse model of Huntington's disease.J Neurosci Res. 2016 Dec;94(12):1400-1410. doi: 10.1002/jnr.23889. Epub 2016 Sep 12. J Neurosci Res. 2016. PMID: 27618125 Free PMC article.
-
Genetic rescue of CB1 receptors on medium spiny neurons prevents loss of excitatory striatal synapses but not motor impairment in HD mice.Neurobiol Dis. 2014 Nov;71:140-50. doi: 10.1016/j.nbd.2014.08.009. Epub 2014 Aug 15. Neurobiol Dis. 2014. PMID: 25134728 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
