Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome
- PMID: 23616559
- PMCID: PMC3684185
- DOI: 10.1523/JNEUROSCI.2764-12.2013
Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome
Abstract
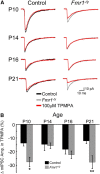
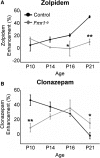
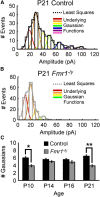
Fragile X syndrome (FXS) is a debilitating neurodevelopmental disorder thought to arise from disrupted synaptic communication in several key brain regions, including the amygdala, a central processing center for information with emotional and social relevance. Recent studies reveal defects in both excitatory and inhibitory neurotransmission in mature amygdala circuits in Fmr1(-/y) mutants, the animal model of FXS. However, whether these defects are the result of altered synaptic development or simply faulty mature circuits remains unknown. Using a combination of electrophysiological and genetic approaches, we show the development of both presynaptic and postsynaptic components of inhibitory neurotransmission in the FXS amygdala is dynamically altered during critical stages of neural circuit formation. Surprisingly, we observe that there is a homeostatic correction of defective inhibition, which, despite transiently restoring inhibitory synaptic efficacy to levels at or beyond those of control, ultimately fails to be maintained. Using inhibitory interneuron-specific conditional knock-out and rescue mice, we further reveal that fragile X mental retardation protein function in amygdala inhibitory microcircuits can be segregated into distinct presynaptic and postsynaptic components. Collectively, these studies reveal a previously unrecognized complexity of disrupted neuronal development in FXS and therefore have direct implications for establishing novel temporal and region-specific targeted therapies to ameliorate core amygdala-based behavioral symptoms.
Figures





Similar articles
-
Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome.J Neurosci. 2010 Jul 21;30(29):9929-38. doi: 10.1523/JNEUROSCI.1714-10.2010. J Neurosci. 2010. PMID: 20660275 Free PMC article.
-
Disrupted inhibitory plasticity and homeostasis in Fragile X syndrome.Neurobiol Dis. 2020 Aug;142:104959. doi: 10.1016/j.nbd.2020.104959. Epub 2020 Jun 6. Neurobiol Dis. 2020. PMID: 32512151 Free PMC article.
-
Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome.J Neurosci. 2011 Jul 27;31(30):10971-82. doi: 10.1523/JNEUROSCI.2021-11.2011. J Neurosci. 2011. PMID: 21795546 Free PMC article.
-
Fragile X syndrome: the GABAergic system and circuit dysfunction.Dev Neurosci. 2011;33(5):349-64. doi: 10.1159/000329420. Epub 2011 Sep 21. Dev Neurosci. 2011. PMID: 21934270 Free PMC article. Review.
-
Amygdala regulation of fear and emotionality in fragile X syndrome.Dev Neurosci. 2011;33(5):365-78. doi: 10.1159/000329424. Epub 2011 Sep 1. Dev Neurosci. 2011. PMID: 21893939 Free PMC article. Review.
Cited by
-
Homeostatic plasticity in neural development.Neural Dev. 2018 Jun 1;13(1):9. doi: 10.1186/s13064-018-0105-x. Neural Dev. 2018. PMID: 29855353 Free PMC article. Review.
-
Dysregulated Prefrontal Cortex Inhibition in Prepubescent and Adolescent Fragile X Mouse Model.Front Mol Neurosci. 2020 May 26;13:88. doi: 10.3389/fnmol.2020.00088. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32528248 Free PMC article.
-
ER stress-induced modulation of neural activity and seizure susceptibility is impaired in a fragile X syndrome mouse model.Neurobiol Dis. 2021 Oct;158:105450. doi: 10.1016/j.nbd.2021.105450. Epub 2021 Jul 23. Neurobiol Dis. 2021. PMID: 34303799 Free PMC article.
-
Fragile X mental retardation protein knockdown in the developing Xenopus tadpole optic tectum results in enhanced feedforward inhibition and behavioral deficits.Neural Dev. 2016 Aug 8;11(1):14. doi: 10.1186/s13064-016-0069-7. Neural Dev. 2016. PMID: 27503008 Free PMC article.
-
Mechanisms Driving the Emergence of Neuronal Hyperexcitability in Fragile X Syndrome.Int J Mol Sci. 2022 Jun 5;23(11):6315. doi: 10.3390/ijms23116315. Int J Mol Sci. 2022. PMID: 35682993 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
