Chronic pain: lost inhibition?
- PMID: 23616562
- PMCID: PMC6619566
- DOI: 10.1523/JNEUROSCI.0174-13.2013
Chronic pain: lost inhibition?
Abstract
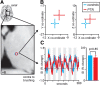
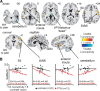
Human brain imaging has revealed that acute pain results from activation of a network of brain regions, including the somatosensory, insular, prefrontal, and cingulate cortices. In contrast, many investigations report little or no alteration in brain activity associated with chronic pain, particularly neuropathic pain. It has been hypothesized that neuropathic pain results from misinterpretation of thalamocortical activity, and recent evidence has revealed altered thalamocortical rhythm in individuals with neuropathic pain. Indeed, it was suggested nearly four decades ago that neuropathic pain may be maintained by a discrete central generator, possibly within the thalamus. In this investigation, we used multiple brain imaging techniques to explore central changes in subjects with neuropathic pain of the trigeminal nerve resulting in most cases (20 of 23) from a surgical event. Individuals with chronic neuropathic pain displayed significant somatosensory thalamus volume loss (voxel-based morphometry) which was associated with decreased thalamic reticular nucleus and primary somatosensory cortex activity (quantitative arterial spin labeling). Furthermore, thalamic inhibitory neurotransmitter content was significantly reduced (magnetic resonance spectroscopy), which was significantly correlated to the degree of functional connectivity between the somatosensory thalamus and cortical regions including the primary and secondary somatosensory cortices, anterior insula, and cerebellar cortex. These data suggest that chronic neuropathic pain is associated with altered thalamic anatomy and activity, which may result in disturbed thalamocortical circuits. This disturbed thalamocortical activity may result in the constant perception of pain.
Figures




Similar articles
-
Chronic Neuropathic Pain: It's about the Rhythm.J Neurosci. 2016 Jan 20;36(3):1008-18. doi: 10.1523/JNEUROSCI.2768-15.2016. J Neurosci. 2016. PMID: 26791228 Free PMC article.
-
Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder.Pain. 2014 Mar;155(3):467-475. doi: 10.1016/j.pain.2013.11.008. Epub 2013 Nov 21. Pain. 2014. PMID: 24269492
-
Altered regional brain T2 relaxation times in individuals with chronic orofacial neuropathic pain.Neuroimage Clin. 2018 Apr 13;19:167-173. doi: 10.1016/j.nicl.2018.04.015. eCollection 2018. Neuroimage Clin. 2018. PMID: 30035014 Free PMC article.
-
Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain.Neuroimage. 2011 Oct 15;58(4):1070-80. doi: 10.1016/j.neuroimage.2011.07.022. Epub 2011 Jul 20. Neuroimage. 2011. PMID: 21798355 Free PMC article. Review.
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
Cited by
-
Exploring Functional Connectivity in Chronic Spinal Cord Injury Patients With Neuropathic Pain Versus Without Neuropathic Pain.Neurotrauma Rep. 2024 Jan 5;5(1):16-27. doi: 10.1089/neur.2023.0070. eCollection 2024. Neurotrauma Rep. 2024. PMID: 38249324 Free PMC article.
-
Altered GABAA receptor function in women with endometriosis: a possible pain-related mechanism.Acta Obstet Gynecol Scand. 2023 Oct;102(10):1316-1322. doi: 10.1111/aogs.14559. Epub 2023 Mar 21. Acta Obstet Gynecol Scand. 2023. PMID: 36944570 Free PMC article.
-
Gray matter alterations in chronic pain: A network-oriented meta-analytic approach.Neuroimage Clin. 2014 Apr 16;4:676-86. doi: 10.1016/j.nicl.2014.04.007. eCollection 2014. Neuroimage Clin. 2014. PMID: 24936419 Free PMC article.
-
Lateral thalamic control of nociceptive response after whisker pad injection of varicella zoster virus.Neuroscience. 2017 Jul 25;356:207-216. doi: 10.1016/j.neuroscience.2017.05.030. Epub 2017 May 24. Neuroscience. 2017. PMID: 28549561 Free PMC article.
-
Structural brain alterations and changes in resting-state functional connectivity in patients with trigeminal neuralgia: A meta-analysis.Neuroimage Clin. 2025;46:103759. doi: 10.1016/j.nicl.2025.103759. Epub 2025 Feb 27. Neuroimage Clin. 2025. PMID: 40086208 Free PMC article. Review.
References
-
- Davis KD, Kiss ZH, Tasker RR, Dostrovsky JO. Thalamic stimulation-evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. J Neurophysiol. 1996;75:1026–1037. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
