Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow
- PMID: 23616566
- PMCID: PMC3714575
- DOI: 10.1096/fj.12-224824
Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow
Abstract
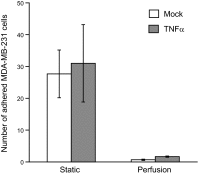
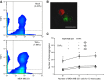
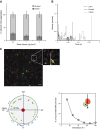
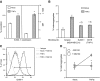
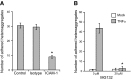
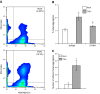
Endothelial adhesion is necessary for the hematogenous dissemination of tumor cells. However, the metastatic breast tumor cell MDA-MB-231 does not bind to the endothelium under physiological flow conditions, suggesting alternate mechanisms of adhesion. Since monocytes are highly represented in the tumor microenvironment, and also bind to endothelium during inflammation, we hypothesized that the monocytes assist in the arrest of MDA-MB-231 on the endothelium. Using in vitro models of the dynamic shear environment of the vasculature, we show that TNF-α-activated THP1/primary human monocytes and MDA-MB-231 cells form stable aggregates, and that the monocytes in these aggregates mediate the adhesion of otherwise nonadherent MDA-MB-231 cells to inflamed endothelium under flow (55±2.4 vs. 1.7±0.82 at a shear stress of 0.5 dyn/cm(2), P<0.01). We also show that the hydrodynamic forces determine the size and orientation of aggregates adhered to the endothelium, and strongly favor the attachment of small aggregates with tumor cells downstream of flow (74-86% doublets at 0.5-2 dyn/cm(2), P<0.01). The 5-fold up-regulation of ICAM-1 on TNF-α-activated MDA-MB-231 cells through the Nf-κB pathway was found to be critical in MDA-MB-231-monocyte aggregation and endothelial adhesion. Our results demonstrate that, under inflammatory conditions, monocytes may serve to disseminate tumor cells through circulation, and the tumor-monocyte-endothelial axis may represent a new therapeutic target to reduce cancer metastasis.
Keywords: MDA-MB-231; aggregation; shear.
Figures


Similar articles
-
Monocyte arrest and transmigration on inflamed endothelium in shear flow is inhibited by adenovirus-mediated gene transfer of IkappaB-alpha.Blood. 1999 Jun 1;93(11):3685-93. Blood. 1999. PMID: 10339475
-
Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway.J Nutr Biochem. 2015 Mar;26(3):293-302. doi: 10.1016/j.jnutbio.2014.11.008. Epub 2014 Dec 15. J Nutr Biochem. 2015. PMID: 25577468 Free PMC article.
-
CRP promotes monocyte-endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions.Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1170-6. doi: 10.1152/ajpheart.00150.2006. Epub 2006 Apr 7. Am J Physiol Heart Circ Physiol. 2006. PMID: 16603696
-
Lipopolysaccharide induces the interactions of breast cancer and endothelial cells via activated monocytes.Cancer Lett. 2014 Apr 1;345(1):75-84. doi: 10.1016/j.canlet.2013.11.022. Epub 2013 Dec 11. Cancer Lett. 2014. PMID: 24333719
-
Computational models of cancer cell transport through the microcirculation.Biomech Model Mechanobiol. 2021 Aug;20(4):1209-1230. doi: 10.1007/s10237-021-01452-6. Epub 2021 Mar 25. Biomech Model Mechanobiol. 2021. PMID: 33765196 Review.
Cited by
-
Modified Systemic Inflammation Score Is an Independent Predictor of Long-Term Outcome in Patients Undergoing Surgery for Adenocarcinoma of the Esophagogastric Junction.Front Surg. 2021 Nov 8;8:622821. doi: 10.3389/fsurg.2021.622821. eCollection 2021. Front Surg. 2021. PMID: 34820414 Free PMC article.
-
Biomechanics in the tumor microenvironment: from biological functions to potential clinical applications.Exp Hematol Oncol. 2025 Jan 11;14(1):4. doi: 10.1186/s40164-024-00591-7. Exp Hematol Oncol. 2025. PMID: 39799341 Free PMC article. Review.
-
Immune Cell Migration to Cancer.Cells. 2024 May 16;13(10):844. doi: 10.3390/cells13100844. Cells. 2024. PMID: 38786066 Free PMC article. Review.
-
Prognostic importance of the preoperative modified systemic inflammation score for patients with gastric cancer.Gastric Cancer. 2019 Mar;22(2):403-412. doi: 10.1007/s10120-018-0854-6. Epub 2018 Jul 7. Gastric Cancer. 2019. PMID: 29982861
-
The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer.Br J Cancer. 2014 Jan 21;110(2):435-40. doi: 10.1038/bjc.2013.785. Epub 2013 Dec 19. Br J Cancer. 2014. PMID: 24357796 Free PMC article.
References
-
- Konstantopoulos K., Thomas S. N. (2009) Cancer cells in transit: the vascular interactions of tumor cells. Annu. Rev. Biomed. Eng. 11, 177–202 - PubMed
-
- Jadhav S., Bochner B., Konstantopoulos K. (2001) Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J. Immunol. 167, 5986–5993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

