Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma
- PMID: 23616570
- PMCID: PMC3891840
- DOI: 10.4049/jimmunol.1202005
Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma
Abstract
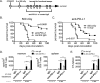
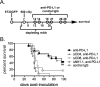
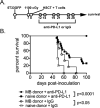
Early phase clinical trials targeting the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers. This pathway appears to play an important role in the failure of immune reactivity to malignant plasma cells in multiple myeloma patients, as the tumor cells express relatively high levels of PD-L1, and T cells show increased PD-1 expression. In the current study, we demonstrate that PD-1/PD-L1 blockade with a PD-L1-specific Ab elicits rejection of a murine myeloma when combined with lymphodepleting irradiation. This particular combined approach by itself has not previously been shown to be efficacious in other tumor models. The antitumor effect of lymphodepletion/anti-PD-L1 therapy was most robust when tumor Ag-experienced T cells were present either through cell transfer or survival after nonmyeloablative irradiation. In vivo depletion of CD4 or CD8 T cells completely eliminated antitumor efficacy of the lymphodepletion/anti-PD-L1 therapy, indicating that both T cell subsets are necessary for tumor rejection. Elimination of myeloma by T cells occurs relatively quickly as tumor cells in the bone marrow were nearly nondetectable by 5 d after the first anti-PD-L1 treatment, suggesting that antimyeloma reactivity is primarily mediated by preactivated T cells, rather than newly generated myeloma-reactive T cells. Anti-PD-L1 plus lymphodepletion failed to improve survival in two solid tumor models, but demonstrated significant efficacy in two hematologic malignancy models. In summary, our results support the clinical testing of lymphodepletion and PD-1/PD-L1 blockade as a novel approach for improving the survival of patients with multiple myeloma.
Figures




Similar articles
-
Durvalumab Combined with Immunomodulatory Drugs (IMiD) Overcomes Suppression of Antitumor Responses due to IMiD-induced PD-L1 Upregulation on Myeloma Cells.Mol Cancer Ther. 2021 Jul;20(7):1283-1294. doi: 10.1158/1535-7163.MCT-20-0246. Epub 2021 Apr 20. Mol Cancer Ther. 2021. PMID: 33879556
-
Bone marrow-derived mesenchymal stem cells inhibit CD8+ T cell immune responses via PD-1/PD-L1 pathway in multiple myeloma.Clin Exp Immunol. 2021 Jul;205(1):53-62. doi: 10.1111/cei.13594. Epub 2021 May 7. Clin Exp Immunol. 2021. PMID: 33735518 Free PMC article.
-
Co-immunizing with PD-L1 induces CD8+ DCs-mediated anti-tumor immunity in multiple myeloma.Int Immunopharmacol. 2020 Jul;84:106516. doi: 10.1016/j.intimp.2020.106516. Epub 2020 Apr 22. Int Immunopharmacol. 2020. PMID: 32334387
-
Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer.Med Mol Morphol. 2017 Dec;50(4):185-194. doi: 10.1007/s00795-017-0170-y. Epub 2017 Sep 21. Med Mol Morphol. 2017. PMID: 28936553 Review.
-
Promises and Pitfalls in the Use of PD-1/PD-L1 Inhibitors in Multiple Myeloma.Front Immunol. 2018 Nov 27;9:2749. doi: 10.3389/fimmu.2018.02749. eCollection 2018. Front Immunol. 2018. PMID: 30538704 Free PMC article. Review.
Cited by
-
Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy.Blood. 2015 Nov 26;126(22):2475-8. doi: 10.1182/blood-2015-03-632919. Epub 2015 Oct 14. Blood. 2015. PMID: 26468228 Free PMC article. Clinical Trial.
-
Immune checkpoint blockade for hematologic malignancies: a review.Stem Cell Investig. 2017 Apr 19;4:32. doi: 10.21037/sci.2017.03.04. eCollection 2017. Stem Cell Investig. 2017. PMID: 28529947 Free PMC article. Review.
-
CAR T-cell immunotherapy: a powerful weapon for fighting hematological B-cell malignancies.Front Med. 2021 Dec;15(6):783-804. doi: 10.1007/s11684-021-0904-z. Epub 2021 Dec 18. Front Med. 2021. PMID: 34921673 Review.
-
Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update.Drugs. 2018 Jan;78(1):19-37. doi: 10.1007/s40265-017-0841-y. Drugs. 2018. PMID: 29188449 Free PMC article. Review.
-
Deregulated Expression of Immune Checkpoints on Circulating CD4 T Cells May Complicate Clinical Outcome and Response to Treatment with Checkpoint Inhibitors in Multiple Myeloma Patients.Int J Mol Sci. 2021 Aug 27;22(17):9298. doi: 10.3390/ijms22179298. Int J Mol Sci. 2021. PMID: 34502204 Free PMC article.
References
-
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. - PubMed
-
- Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased Level of both CD4+FOXP3+ Regulatory T Cells and CD14+HLA-DR(−)/low Myeloid-Derived Suppressor Cells and Decreased Level of Dendritic Cells in Patients with Multiple Myeloma. Scand J Immunol. 2010;72:540–547. - PubMed
-
- Sharabi A, Ghera NH. Breaking tolerance in a mouse model of multiple myeloma by chemoimmunotherapy. Adv Cancer Res. 2010;107:1–37. - PubMed
-
- Hallett WHD, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17:1133–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

