Galectin 1 modulates plasma cell homeostasis and regulates the humoral immune response
- PMID: 23616571
- PMCID: PMC3660186
- DOI: 10.4049/jimmunol.1201885
Galectin 1 modulates plasma cell homeostasis and regulates the humoral immune response
Abstract
Galectin-1 (GAL1) is an S-type lectin with multiple functions, including the control of B cell homeostasis. GAL1 expression was reported to be under the control of the plasma cell master regulator BLIMP-1. GAL1 was detected at the protein level in LPS-stimulated B cells and was shown to promote Ig secretion in vitro. However, the pattern of GAL1 expression and function of GAL1 in B cells in vivo are still unclear. In this study, we show that, among B cells, GAL1 is only expressed by differentiating plasma cells following T-dependent or T-independent immunization. Using GAL1-deficient mice we demonstrate that GAL1 expression is required for the maintenance of Ag-specific Ig titers and Ab-secreting cell numbers. Using an in vitro differentiation assay we find that GAL1-deficient plasmablasts can develop normally but die rapidly, through caspase 8 activation, under serum starvation-induced death conditions. TUNEL assays show that in vivo-generated GAL1-deficient plasma cells exhibit an increased sensitivity to apoptosis. Taken together, our data indicate that endogenous GAL1 supports plasma cell survival and participates in the regulation of the humoral immune response.
Figures

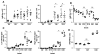



References
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Bourne Y, Bolgiano B, Liao DI, Strecker G, Cantau P, Herzberg O, Feizi T, Cambillau C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat struct biol. 1994;1:863–870. - PubMed
-
- Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

