The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals
- PMID: 23616581
- PMCID: PMC4151560
- DOI: 10.2967/jnumed.112.115550
The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals
Abstract
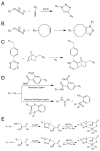
Click chemistry has become a ubiquitous chemical tool with applications in nearly all areas of modern chemistry, including drug discovery, bioconjugation, and nanoscience. Radiochemistry is no exception, as the canonical Cu(I)-catalyzed azide-alkyne cycloaddition, strain-promoted azide-alkyne cycloaddition, inverse electron demand Diels-Alder reaction, and other types of bioorthogonal click ligations have had a significant impact on the synthesis and development of radiopharmaceuticals. This review will focus on recent applications of click chemistry ligations in the preparation of imaging agents for SPECT and PET, including small molecules, peptides, and proteins labeled with radionuclides such as (18)F, (64)Cu, (111)In, and (99m)Tc.
Keywords: click chemistry; radiochemistry.
Figures


References
-
- Best MD. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochem. 2009;48:6571–6584. - PubMed
-
- Waengler C, Schirrmacher R, Bartenstein P, Waengler B. Click-chemistry reactions in radiopharmaceutical chemistry: Fast & easy introduction of radiolabels into biomolecules for in vivo imaging. Curr Med Chem. 2010;17:1092–1116. - PubMed
-
- Hausner SH, Marik J, Gagnon MKJ, Sutcliffe JL. In vivo positron emission tomography (pet) imaging with an alphavbeta6 specific peptide radiolabeled using 18F-“click” chemistry: Evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem. 2008;51:5901–5904. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
