Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial
- PMID: 23616624
- PMCID: PMC3674665
- DOI: 10.1182/blood-2012-11-464503
Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial
Abstract
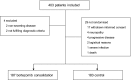
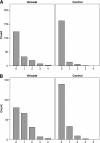
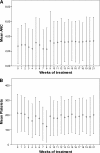
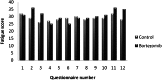
The Nordic Myeloma Study Group conducted an open randomized trial to compare bortezomib as consolidation therapy given after high-dose therapy and autologous stem cell transplantation (ASCT) with no consolidation in bortezomib-naive patients with newly diagnosed multiple myeloma. Overall, 370 patients were centrally randomly assigned 3 months after ASCT to receive 20 doses of bortezomib given during 21 weeks or no consolidation. The hypothesis was that consolidation therapy would prolong progression-free survival (PFS). The PFS after randomization was 27 months for the bortezomib group compared with 20 months for the control group (P = .05). Fifty-one of 90 patients in the treatment group compared with 32 of 90 controls improved their response after randomization (P = .007). No difference in overall survival was seen. Fatigue was reported more commonly by the bortezomib-treated patients in self-reported quality-of-life (QOL) questionnaires, whereas no other major differences in QOL were recorded between the groups. Consolidation therapy seemed to be beneficial for patients not achieving at least a very good partial response (VGPR) but not for patients in the ≥ VGPR category at randomization. Consolidation with bortezomib after ASCT in bortezomib-naive patients improves PFS without interfering with QOL. This trial was registered at www.clinicaltrials.gov as #NCT00417911.
Figures

References
-
- Attal M, Harousseau JL, Stoppa AM, et al. Intergroupe Français du Myélome. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335(2):91–97. - PubMed
-
- Lenhoff S, Hjorth M, Holmberg E, et al. Nordic Myeloma Study Group. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study [published correction appears in Blood. 2010;116(13):2402]. Blood. 2000;95(1):7–11. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. - PubMed
-
- Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 2011;29(14):1898–1906. - PubMed
-
- Mellqvist UH, Lenhoff S, Johnsen HE, et al. Nordic Myeloma Study Group. Cyclophosphamide plus dexamethasone is an efficient initial treatment before high-dose melphalan and autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of a randomized comparison with vincristine, doxorubicin, and dexamethasone. Cancer. 2008;112(1):129–135. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

