Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice
- PMID: 23616627
- PMCID: PMC3661714
- DOI: 10.1161/CIRCULATIONAHA.112.000116
Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice
Abstract
Background: Exaggerated and prolonged inflammation after myocardial infarction (MI) accelerates left ventricular remodeling. Inflammatory pathways may present a therapeutic target to prevent post-MI heart failure. However, the appropriate magnitude and timing of interventions are largely unknown, in part because noninvasive monitoring tools are lacking. Here, we used nanoparticle-facilitated silencing of CCR2, the chemokine receptor that governs inflammatory Ly-6C(high) monocyte subset traffic, to reduce infarct inflammation in apolipoprotein E-deficient (apoE(-/-)) mice after MI. We used dual-target positron emission tomography/magnetic resonance imaging of transglutaminase factor XIII (FXIII) and myeloperoxidase (MPO) activity to monitor how monocyte subset-targeted RNAi altered infarct inflammation and healing.
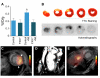
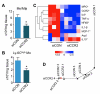
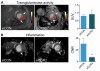
Methods and results: Flow cytometry, gene expression analysis, and histology revealed reduced monocyte numbers and enhanced resolution of inflammation in infarcted hearts of apoE(-/-) mice that were treated with nanoparticle-encapsulated siRNA. To follow extracellular matrix cross-linking noninvasively, we developed a fluorine-18-labeled positron emission tomography agent ((18)F-FXIII). Recruitment of MPO-rich inflammatory leukocytes was imaged with a molecular magnetic resonance imaging sensor of MPO activity (MPO-Gd). Positron emission tomography/magnetic resonance imaging detected anti-inflammatory effects of intravenous nanoparticle-facilitated siRNA therapy (75% decrease of MPO-Gd signal; P<0.05), whereas (18)F-FXIII positron emission tomography reflected unimpeded matrix cross-linking in the infarct. Silencing of CCR2 during the first week after MI improved ejection fraction on day 21 after MI from 29% to 35% (P<0.05).
Conclusion: CCR2-targeted RNAi reduced recruitment of Ly-6C(high) monocytes, attenuated infarct inflammation, and curbed post-MI left ventricular remodeling.
Keywords: RNA, small interfering; heart failure; inflammation; magnetic resonance imaging; molecular imaging; monocytes; myocardial infarction; positron-emission tomography; ventricular remodeling.
Figures





Comment in
-
Blinding the monocytes to protect the heart.Circulation. 2013 May 21;127(20):2006-8. doi: 10.1161/CIRCULATIONAHA.113.003045. Epub 2013 Apr 24. Circulation. 2013. PMID: 23616628 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

