The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase
- PMID: 23616652
- PMCID: PMC3700292
- DOI: 10.1128/JVI.00632-13
The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase
Abstract
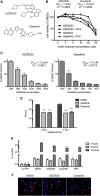
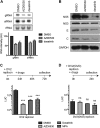
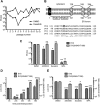
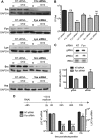
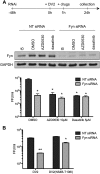
In this study, we characterized the antiviral mechanism of action of AZD0530 and dasatinib, two pharmacological inhibitors of host kinases, that also inhibit dengue virus (DV) infection. Using Northern blot and reporter replicon assays, we demonstrated that both small molecules inhibit the DV2 infectious cycle at the step of steady-state RNA replication. In order to identify the cellular target of AZD0530 and dasatinib mediating this anti-DV2 activity, we examined the effects of RNA interference (RNAi)-mediated depletion of the major kinases known to be inhibited by these small molecules. We determined that Fyn kinase, a target of both AZD0530 and dasatinib, is involved in DV2 RNA replication and is probably a major mediator of the anti-DV activity of these compounds. Furthermore, serial passaging of DV2 in the presence of dasatinib led to the identification of a mutation in the transmembrane domain 3 of the NS4B protein that overcomes the inhibition of RNA replication by AZD0530, dasatinib, and Fyn RNAi. Although we observed that dasatinib also inhibits DV2 particle assembly and/or secretion, this activity does not appear to be mediated by Src-family kinases. Together, our results suggest that AZD0530 and dasatinib inhibit DV at the step of viral RNA replication and demonstrate a critical role for Fyn kinase in this viral process. The antiviral activity of these compounds in vitro makes them useful pharmacological tools to validate Fyn or other host kinases as anti-DV targets in vivo.
Figures

References
-
- Wengler G, Wengler G. 1993. The NS 3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology 197:265–273 - PubMed
-
- Wengler G, Czaya G, Färber PM, Hegemann JH. 1991. In vitro synthesis of West Nile virus proteins indicates that the amino-terminal segment of the NS3 protein contains the active centre of the protease which cleaves the viral polyprotein after multiple basic amino acids. J. Gen. Virol. 72(Pt 4):851–858 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

