Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design
- PMID: 23616655
- PMCID: PMC3700278
- DOI: 10.1128/JVI.03577-12
Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design
Abstract
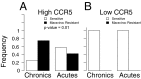
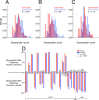
Understanding human immunodeficiency virus type 1 (HIV-1) transmission is central to developing effective prevention strategies, including a vaccine. We compared phenotypic and genetic variation in HIV-1 env genes from subjects in acute/early infection and subjects with chronic infections in the context of subtype C heterosexual transmission. We found that the transmitted viruses all used CCR5 and required high levels of CD4 to infect target cells, suggesting selection for replication in T cells and not macrophages after transmission. In addition, the transmitted viruses were more likely to use a maraviroc-sensitive conformation of CCR5, perhaps identifying a feature of the target T cell. We confirmed an earlier observation that the transmitted viruses were, on average, modestly underglycosylated relative to the viruses from chronically infected subjects. This difference was most pronounced in comparing the viruses in acutely infected men to those in chronically infected women. These features of the transmitted virus point to selective pressures during the transmission event. We did not observe a consistent difference either in heterologous neutralization sensitivity or in sensitivity to soluble CD4 between the two groups, suggesting similar conformations between viruses from acute and chronic infection. However, the presence or absence of glycosylation sites had differential effects on neutralization sensitivity for different antibodies. We suggest that the occasional absence of glycosylation sites encoded in the conserved regions of env, further reduced in transmitted viruses, could expose specific surface structures on the protein as antibody targets.
Figures



References
-
- Shaw GM, Hunter E. 2012. HIV transmission, p 135–157 In Bushman FD, Nabel GJ, Swanstrom R. (ed), HIV: from biology to prevention and treatment. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, Salazar-Gonzalez JF, Bhattacharya T, Chu H, Hoffman I, Galvin S, Mapanje C, Kazembe P, Thebus R, Fiscus S, Hide W, Cohen MS, Karim SA, Haynes BF, Shaw GM, Hahn BH, Korber BT, Swanstrom R, Williamson C. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556–3567 - PMC - PubMed
-
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022 - PubMed
-
- Fischer W, Ganusov VV, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo CC, Nag A, Wallstrom TC, Wang S, McMichael AJ, Haynes BF, Hahn BH, Perelson AS, Borrow P, Shaw GM, Bhattacharya T, Korber BT. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303.10.1371/journal.pone.0012303 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

