Early Gag immunodominance of the HIV-specific T-cell response during acute/early infection is associated with higher CD8+ T-cell antiviral activity and correlates with preservation of the CD4+ T-cell compartment
- PMID: 23616666
- PMCID: PMC3700299
- DOI: 10.1128/JVI.00865-13
Early Gag immunodominance of the HIV-specific T-cell response during acute/early infection is associated with higher CD8+ T-cell antiviral activity and correlates with preservation of the CD4+ T-cell compartment
Abstract
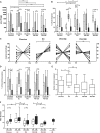
The important role of the CD8(+) T-cell response on HIV control is well established. Moreover, the acute phase of infection represents a proper scenario to delineate the antiviral cellular functions that best correlate with control. Here, multiple functional aspects (specificity, ex vivo viral inhibitory activity [VIA] and polyfunctionality) of the HIV-specific CD8(+) T-cell subset arising early after infection, and their association with disease progression markers, were examined. Blood samples from 44 subjects recruited within 6 months from infection (primary HIV infection [PHI] group), 16 chronically infected subjects, 11 elite controllers (EC), and 10 healthy donors were obtained. Results indicated that, although Nef dominated the anti-HIV response during acute/early infection, a higher proportion of early anti-Gag T cells correlated with delayed progression. Polyfunctional HIV-specific CD8(+) T cells were detected at early time points but did not associate with virus control. Conversely, higher CD4(+) T-cell set points were observed in PHI subjects with higher HIV-specific CD8(+) T-cell VIA at baseline. Importantly, VIA levels correlated with the magnitude of the anti-Gag cellular response. The advantage of Gag-specific cells may result from their enhanced ability to mediate lysis of infected cells (evidenced by a higher capacity to degranulate and to mediate VIA) and to simultaneously produce IFN-γ. Finally, Gag immunodominance was associated with elevated plasma levels of interleukin 2 (IL-2) and macrophage inflammatory protein 1β (MIP-1β). All together, this study underscores the importance of CD8(+) T-cell specificity in the improved control of disease progression, which was related to the capacity of Gag-specific cells to mediate both lytic and nonlytic antiviral mechanisms at early time points postinfection.
Figures






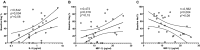
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 - PubMed
-
- McDermott AB, Koup RA. 2012. CD8(+) T cells in preventing HIV infection and disease. AIDS 26:1281–1292 - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205–211 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

