Clinical evaluation of zaleplon in the treatment of insomnia
- PMID: 23616704
- PMCID: PMC3630939
- DOI: 10.2147/nss.s6853
Clinical evaluation of zaleplon in the treatment of insomnia
Abstract
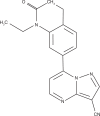
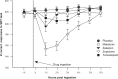
Zaleplon is a pyrazolopyrimidine hypnotic used for the treatment of insomnia. Zaleplon binds preferentially at the α1β2γ2 subunit of gamma aminobutyric acid type A (GABAA) receptors in the central nervous system, and has a half-life of about one hour. Efficacy studies show that zaleplon is a suitable hypnotic for sleep initiation purposes. However, because of its short half-life, zaleplon is less effective in sleep maintenance when compared with other hypnotics. Nevertheless, zaleplon does increase total sleep time. No rebound effects are observed after treatment discontinuation. The use of zaleplon is relatively safe. Adverse effects are mild and of short duration. No important interactions have been reported, and there is no evidence of abuse potential. Relative to benzodiazepine hypnotics, the biggest advantage of zaleplon is that current evidence suggests it does not produce residual next-day effects. As early as four hours after intake of zaleplon, no effects on cognitive, memory, psychomotor performance, and the ability to drive a car have been reported. Future studies should confirm these findings, and comparisons with new nonbenzodiazepine hypnotics should determine the importance of zaleplon in the future treatment of insomnia.
Keywords: efficacy; hypnotics; residual effects; safety; zaleplon; zolpidem.
Figures

References
-
- Verster JC, Pandi-Perumal SR, Streiner DL. Sleep and Quality of Life in Clinical Medicine. New York, NY: Humana Press (Springer); 2008.
-
- Pandi-Perumal SR, Verster JC, Monti JM, Lader MH, Langer SZ. Sleep Disorders: Diagnosis and Therapeutics. London: Informa Healthcare (Taylor and Francis); 2008.
-
- Ebben MR, Spielman AJ. Non-pharmacological treatments for insomnia. J Behav Med. 2009;32(3):244–254. - PubMed
-
- Barbera J, Shapiro C. Benefit-risk assessment of zaleplon in the treatment of insomnia. Drug Saf. 2005;28(4):301–318. - PubMed
-
- Dooley M, Plosker GL. Zaleplon: A review of its use in the treatment of insomnia. Drugs. 2000;60(2):413–445. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

